Abstract
Introduction
Soft tissue fillers are widely used and are commonly considered to be safe. Nonetheless, adverse events such as late inflammatory reactions (LIRs) are reported for every type of filler. As of the start of the COVID-19 pandemic, LIRs have been reported after SARS-CoV infection or vaccination. In the past, we reviewed these adverse events; however, since then, we faced a wave with the Omicron, and the vaccination programs continued with booster vaccines. We therefore aimed to perform an up-to-date review of the literature on LIRs after COVID-19 infection and vaccination with additional learned lessons from this pandemic.
Material and methods
We performed a systematic review on soft tissue filler-related LIRs after SARS-CoV-2 infection or vaccination in line with the PRISMA guidelines. Eligible studies were searched in the database PubMed from 1 August 2021 until 1 June 2023. Data on patient characteristics, filler characteristics, clinical findings, and treatment options were retrieved.
Results
A total of 14 papers with in total 52 patients were reported, of which 16 had adverse events after a SARS-CoV-2 infection and 36 after SARS-CoV-2 vaccination. In most cases, it concerned females who had their (mostly temporary) fillers for cosmetic purposes. Symptoms were reported in a matter of hours up to weeks after SARS-CoV-2 vaccination (22 Pfizer, 7 Moderna, 3 AstraZeneca, 3 Sputnik V, and one after Siophram), mostly after the first or second dose but sporadically after a third dose. Most patients were treated in a conservative manner.
Discussion
LIRs continue to be reported after SARS-CoV-2 infection and vaccination and are currently also reported for non-mRNA vaccines, for non-temporary fillers, and also after a third dose of the vaccine. Although there are more and more papers on this matter, they remain minor and self-limiting. We therefore still advise patients with soft tissue fillers to remain participated in vaccination programs when needed.
Level of evidence: Not gradable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of non-surgical cosmetic treatments, such as soft tissue fillers, has grown rapidly over the past decades. According to the American Society of Cosmetic Plastic Surgeons, it is estimated that their use has increased by 144% since the beginning of this decade [1,2,3]. Soft tissue fillers are used for cosmetic purposes as well as for reconstruction, and their popularity has been addressed to their non-invasive character, quick results, and relative low adverse events [4]. Up to date, there have been more than hundreds of products on the market. Although there is not a clear and universal classification for these products, the most used classification is by their biodegradability [5]. According to this classification, they are divided into temporary, biostimulatory, or permanent (Table 1) [6, 7]. Of these different types of fillers, hyaluronic acid (HA) is currently the most used type of filler worldwide [1].
Although the process of material production is more and more advanced, adverse events occur in all products, regardless of their biodegradability [8,9,10,11]. Of all adverse events, late inflammatory reactions (LIRs) are one of the most common reported [12]. They include reactions such as erythematous lumps, granulomas, edema, or nodules and develop between hours up to years after filler infection. It is reported that LIRs occur in 0.01–4.25% after filler injection [13]. Although advances have been made in understanding their etiology, the exact mechanism is still unknown. Several hypotheses have been proposed, such as subclinical infection, foreign body reaction, delayed‐type hypersensitivity reaction, and adjuvant‐based filler reactions due to triggers such as infection, trauma, or vaccination [14,15,16,17,18,19,20,21,22,23,24,25].
It was therefore expected that LIRs would be reported in the COVID-19 pandemic after SARS-CoV-2 infection and vaccination. In the early beginning of investigations on SARS-CoV-2 vaccination, the Food and Drug Administration (FDA) reported on three patients with soft tissue fillers who experienced adverse events after vaccination with Moderna [26]. Hereafter, more and more case reports were published regarding adverse events related to SARS-CoV-2 infection and vaccination [27,28,29,30,31,32]. We therefore previously performed a systematic review on LIRs after SARS-CoV-2 infection and vaccination [33].
In our former review reporting on LIRs from the start of the COVID-19 pandemic (January 2020) up to August 2021, we reported on 7 papers with a total of 19 patients after SARS-CoV infection or vaccination [33]. It concerned 3 cases after infection and 16 cases after vaccination who reported symptoms such as facial swelling or angioedema. In the case of post-vaccination cases, it was mainly reported after Moderna (13 cases) and Pfizer (3 cases), both after the first and second dose from 13 h up to several weeks. In general, the adverse events were minor and self-limiting.
However, since our previous review, we faced a wave with the Omicron, and the vaccination programs continued with booster vaccines [34]. On May 5, 2023, the COVID-19 pandemic was declared no longer to be a public health emergency of international concern [35]. The aim of this review was therefore to provide an up-to-date overview of LIRs after COVID-19 infection with additional learned lessons from this pandemic.
Method
Literature search and selection criteria
We set up a systematic review on LIRs of soft tissue filler use after SARS-CoV-2 infection or vaccination. This review was carried out in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) guidelines [36]. Eligible studies were searched in the database PubMed from 1 August 2021 until 1 June 2023. The search strategy included the terms “filler” and “adverse events” (see supplement data file for the full search strategy). All original studies on soft tissue filler-related LIRs after SARS-CoV-2 infection or vaccination were included. There were no exclusion criteria on patient, soft tissue filler, or study characteristics. We have set up a search strategy with the help of an experienced medical information specialist. The retrieved studies were screened respectively on title, abstract, and lastly on full text.
Data extraction
The following data were extracted from the included studies: authors, year of publication, study design, number of patients, type/amount and location sites of injected filler, age and sex of patients, primary indication for injection, type of complication, vaccine brand, injection duration until SARS-CoV-2 positive or vaccination, and type of treatment. We assessed the level of evidence according to the Oxford Centre for Evidence-Based Medicine [37].
Results
Article and patient demographics
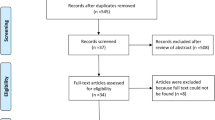
The study selection flow chart is shown in Fig. 1. In total, 1103 studies were identified in PubMed. After screening of the title and abstract, respectively, 136 and 55 article papers were found eligible for inclusion. After reading the full text, we finally included 14 papers (Table 2). These 14 papers described 52 patients with LIRs, of which 16 patients had adverse events after a SARS-CoV-2 infection and 36 patients after SARS-CoV-2 vaccination. These 36 patients had their adverse events after the following vaccinations: 22 after Pfizer, 7 after Moderna, 3 after AstraZeneca, 3 after Sputnik V, and one after Sinophram. In 18 cases, this occurred after the first dose, in 12 cases after the second dose, in two cases after the first as well as the second dose, in two cases after a third dose, and in two cases this was not reported. Most patients had temporary fillers such as hyaluronic acid (n = 34), collagen (n = 9), or a combination of those two (n = 2). But also reactions were seen after biostimulatory (polycaprolactone (n = 1), polymethyl methacrylate (n = 1), and calcium hydroxylapatite (n = 1)) and permanent fillers (fluid silicone (n = 1) and polyalkylimide (n = 1)). In one case, a combination was used (hyaluronic acid and methacrylate), and in one, it was unknown.
LIRs after SARS-CoV-2 infection
Three studies investigated cases with LIRs after SARS-CoV-2 infection (Table 3). Liu et al. present a case of a patient who had her filler 5 years prior to SARS-CoV-2 infection [38]. A month after infection, she developed a subcutaneous nodule in the right perioral area which progressed over time. She therefore had it surgically removed. Pathologic examination showed diffuse infiltrate of granulomatous inflammation in the dermis of numerous non‐necrotizing granulomas which varied in size and shape. The authors postulate that these pathology findings are consistent with foreign body granulomatous reaction to HA. Virdi reported on a 55-year-old female who had hyaluronic injection on several sites of her face without any complications thus far [39]. Eight months after her last injections, she developed COVID-19 symptoms which was then PCR confirmed. Thirteen days hereafter, she developed angioedema of the lower face and lips. She was treated with corticosteroids and high-dose antihistamines after which the swelling and tenderness completely resolved.
Although it is unknown whether the patients included in the paper of Kato et al. have had an infection with SARS-CoV-2, they report on a tremendous increase of LIRs during the COVID-19 pandemic (report from May 2020 and June 2021) compared to the pre-COVID-19 area [40]. LIRs in the 12 years before COVID-19 were only seen in one patient. During the current area, they treated about 1180 patients with soft tissue filler (HA and collagen) where a total of 14 patients developed LIRs mostly of the tear through. This occurred mostly within hours up to a few days. None of the patients was vaccinated during this study as COVID-19 vaccinations were not available yet. In half of the patients, the complications resolved without any intervention, while the other half was treated with prednisolone. The authors suggest that the increase in LIRs during the COVID-19 pandemic might be the result of alterations of the immune system, subclinical SARS-CoV-2 infection, or altered stress levels.
LIRs after SARS-CoV-2 vaccination
Eleven papers with a total of 36 patients with adverse events after the SARS-CoV-2 vaccination were included (Table 4). It mostly concerned LIRs after mRNA vaccines in patients with HA. But for the first time, LIRs after non-mRNA vaccines have been reported, as well as for non-HA fillers.
Cases with HA filler and mRNA vaccines
Michon reported on two patients with complications after Pfizer vaccine from his clinical practice [41]. A 39-year-old female with an HA injection in her tear trough reported an erythematous swelling in just 2 days after her first dose of Pfizer vaccine. After careful examination, a watchful waiting regime was chosen, and the swelling resolved. A second 61-year-old patient also experienced adverse events after her first Pfizer vaccine. It concerned a patient with several facial treatments with HA who experienced intermitted facial swelling at several facial sides a few days after the vaccine. She was seen 3 weeks hereafter at the clinic where she was treated with hyaluronidase after which she was symptom-free. Savva et al. reported on a 38-year-old female who had lip fillers (HA) a month prior to her first Pfizer vaccine [42]. A few days after her first vaccine, she developed small erythematous nodules both on her upper as well as lower lip. These nodules resolved spontaneously within a week. After her second dose, she again developed erythematous nodules, but this time 2 months after her vaccine. She was therefore treated with methylprednisolone after which the nodules disappeared. Osmond et al. published a case report on a 26-year-old female who had HA injection in her cheeks and chin 3 years prior to her Moderna vaccine [43]. There were no reactions reported after her first vaccine; however, she developed complications within 24 h after her second vaccine. She experienced a tremendous enlargement of her chin accompanied by slurred speech, paresthesia of the lower face, headache, and malaise. All symptoms resolved within 28 h without any intervention. Beamish et al. report a case of a 23-year-old who had filler a year prior to her second Pfizer vaccine [44]. Six weeks hereafter, she presented at the emergency room with painful asymmetric swelling over her maxilla, lips, and lower jaw. She had no systemic symptoms and was treated with antihistamine and one dose of dexamethasone. Michon reports on a 45-year-old woman who had hyaluronic fillers in her chin and lips [41]. When receiving her third Pfizer dose, she developed swelling of the lips within 24 h. It is unknown if she had the same reaction for her first and second doses. She was treated with lisinopril for 7 days with good results. Alharithy et al. report on 41- and 31-year-old females, both of whom have had hyaluronic acid injection in the past and who presented with swelling after their Pfizer vaccine [45]. They presented with swelling of the upper lip and eyes and were respectively treated with antihistamines and lisinopril with good response. Azzouz et al. presented a case of complications after a first and second SARS-CoV-2 vaccine [46]. It concerned a 43-year-old woman with multiple hyaluronic acid injections which have been well tolerated. Three weeks after her first dose of the Moderna vaccine, she developed an erythematous pustule in the left cheek where she previously had a filler injection. This pustule remained even after treatment with antibiotics. Three months later, she received her second dose of the Moderna vaccine, and within 24 h, she developed malaise and facial edema. In the upcoming weeks, she devolved new nodules at previous sites of HA injection (cheeks and chin). The patient was treated with prednisone, steroid injections, and hyaluronidase.
Cases with non-HA filler and non-mRNA vaccines
Kalantari et al. presented one of the first case reports of LIRs after a non-hyaluronic acid filler, namely, polycaprolactone (PCL), a biostimulatory filler [47]. A 62-year-old female had a PCL injection of the dorsum of the hand 2 years prior to COVID-19 vaccination. She received her first Sinophram vaccination without any symptoms. However, 2 weeks after her second dose, she developed painless nodules on the dorsum of both hands. She received a single dose of dexamethasone, topical corticosteroid, and intralesional triamcinolone injection in nodules of which the last two mentioned resulted in a significant improvement of the lesions. Alijotas‐Reig et al. reported on 20 patients with adverse events after SARS-CoV-2 vaccination [48]. It concerned 20 females ranging from 21 to 71 years with mostly hyaluronic acid, but also with permanent fillers. Three patients have experienced adverse events previously before vaccination. They currently received diverse vaccinations (11/20 (55%) Pfizer, 5/20 (25%) Moderna, 3/20 (15%) Astra‐Zeneca, and 1/20 (5.0%) Sputnik V) after which 13/20 (65%) cases experienced complications after the first dose and 7/20 (35%) after the second dose. The patients had a wide variety of symptoms such as edema, induration, granuloma, fever, and in 2 cases systemic complaints (myalgia or arthralgia). In 3/20 patients, the symptoms resolved without any intervention, 14/20 had antihistamines (sometimes in combination with non‐steroidal anti‐inflammatory drugs and prednisone, or both), and 3/20 were treated with an unknown regime. In three cases, a full response was not achieved. Ortigosa et al. described a case series of five females treated with hyaluronic acid who received Pfizer or AstraZeneca [49]. They described among others a case of a 35-year-old female who had hyaluronic acid treatment of the lips, nasojugal furrow, malar, and chin region 16 months prior to her AstraZeneca vaccination. Twenty-four hours after the first dose of her vaccine, she presented with adverse events of her lips and chin, for which she was treated with prednisone for 7 days. After 2 days, her symptoms improved significantly. However, adverse events started to occur in other facial regions. In total, she received 21 days steroid treatment with remaining mild erythema. They also describe the case of a female who had edema of her eyelids 1 month after Pfizer vaccination with recurrence at 2 and 4 months. There was no recurrence after the final treatment with lisinopril. Moreover, they also present one of the first cases of a patient who developed edema only after the third dose of the Pfizer vaccine; it concerned a 34-year-old woman with HA treatment of her lips. She developed edema of the lips after receiving the third dose of the Pfizer vaccine. It is not reported how she reacted on the first two doses of this vaccine. She received antihistamine after which she fully recovered. Jeon et al. present a case report of a 47-year-old female who had facial augmentation with calcium hydroxylapatite 14 years prior to vaccination [50]. She reported recurring swelling of the cheeks almost every year that disappears within a few days. She currently reported symptoms just 3 h after her second dose of the Pfizer vaccine. Firm mass-like nodules presented on both cheeks, but other symptoms such as erythema and tenderness were much prominent on the left side. A computed tomography (CT) scan revealed abscesses in both cheeks. As a consequence, the authors performed an incision and drainage at the operating room where a yellow pus-like material came out. Microbial cultures revealed Staphylococcus aureus growth, while pathology reports showed infiltration of inflammatory cells. The patient was treated with a combination of antibiotics and methylprednisolone after which she was fully recovered after 3 weeks.
Discussion
During the first 1.5 years of the COVID-19 pandemic, a handful of case reports were published on adverse events after SARS-CoV-2 infection and mostly after SARS-CoV-2 vaccination. This has led to several questions and concerns among people with those fillers. We previously published a systemic review on this matter [33]. As the COVID-19 pandemic and appurtenant vaccination programs continue, we aimed to give an up-to-date overview of LIRs following SARS-CoV-2 infection and vaccination.
In this second part of our review on adverse events after SARS-CoV infection or vaccination, we found 52 patients in 14 articles of which 16 had adverse events after a SARS-CoV-2 infection and 35 after SARS-CoV-2 vaccination. These post-vaccination cases occurred after vaccination with Pfizer (n = 22), Moderna (n = 7), AstraZeneca (n = 3), Sputnik V (n = 3) after Sinophram (n = 1). In 18 cases, this occurred after the first dose, in 12 cases after the second dose, in two cases after the first as well as the second dose, in two cases after a third dose, and in two cases this was not reported. Most patients had temporary fillers such as hyaluronic acid or collagen (n = 45), but a few reactions were also seen after biostimulatory and permanent fillers. Most patients could be treated in a conservative manner.
If we summarize all patients with LIRs since the beginning of the COVID-19 pandemic found in our first review and the current review, we find a total of 21 papers that reported on a total of 71 patients with adverse events after SARS-CoV-2 infection or vaccination. A total of 19 patients were thought to have LIRs after an infection, while 52 reported on LIRs after vaccination. In the case of post-vaccination LIRs, it mostly concerned vaccination with Pfizer (n = 25) and Moderna (n = 20), both mRNA vaccines. In our former review, Moderna (n = 13) was more dominant than Pfizer (n = 3), but it seems that LIRs related to Pfizer have relatively increased over the time. Also, in the current review, we saw for the first time LIRs after non-mRNA vaccines such as AstraZeneca, Sputnik V, and Sinophram. So far, it was known that LIRs occurred after the first and second doses, but this review also reports on two cases after a third dose. Most patients had temporary fillers such as hyaluronic acid or collagen (n = 52), but a few cases after biostimulatory and permanent fillers were reported for the first time in the current review.
In our previous review, we discussed the role of immunobiological factors in the aetiology of LIRs after SARS-CoV-2 infection and vaccination [33]. These theories include an extensive role for delayed-type hypersensitivity (type IV) reactions as fillers are one of the few materials that can evoke a hypersensitivity (type IV) reaction [51]. However, in the case of LIRs, this is not fully investigated, and more research is needed on this matter. Others suggest that foreign body materials such as fillers might actually act as adjuvants, rather than antigens [22]. Those adjuvants subsequently stimulate an immune response, finally leading to LIRs. It has been suggested that several triggers such as infections and vaccinations might act as adjuvants themselves or might induce adjuvant activity. More recent research also hypothesize that adverse events might be the result of genetic predisposition for an altered immune response against foreign bodies [52]. More research is needed on this matter.
We acknowledge the presence of several limitations within this review. Firstly, the majority of the included studies consist of case reports or case series, which inherently constrains our ability to gain a comprehensive understanding of the issue’s prevalence. The absence of prospective or case–control studies raises the possibility that only the most severe cases come to medical attention, potentially resulting in an underrepresentation of the actual extent of the problem. Additionally, attributing a causal relationship between the reported complications and either SARS-CoV-2 infection or vaccination proves challenging, given that LIRs can also manifest independently subsequent to filler injections. Furthermore, the bulk of post-vaccination complications are linked to the Pfizer and Moderna vaccines. It is important to recognize that these vaccines have achieved widespread global utilization, logically leading to a higher frequency of reported complications associated with their use.
Since we have gained new insights due to this follow-up review concerning soft tissue-related LIRs after SARS-CoV-2 infection and vaccination, we want to provide additional recommendations for clinical practice. First of all, the relationship between SARS-CoV-2 infection, vaccination, and LIRs seems to be underlined as more and more reports have been published on this matter. SARS-CoV-2 infection and vaccination-related LIRs are still mostly minor and self-limiting. Although the COVID-19 pandemic is no longer a public health emergency of international concern, we still recognize the importance of pre-vaccination counseling for any vaccination for patients with dermal fillers concerning allergies and a history of adverse events against any type of foreign body material. In our first review, we only found adverse events in mRNA vaccines (Moderna or Pfizer); however, recently a few case reports have also been reported for non-mRNA vaccines. Although all vaccines induce these adverse events, they are still major for the mRNA vaccines, and we suggest that patients with fillers take other vaccines if possible. We still advise a 2–4 week window between filler injections and vaccination in general and 2 months longer for immunocompromised patients (i.e., patients with immunosuppressive medications, chemotherapy, or immunologic disorders). When adverse events occur, we still advise to give oral steroids as primary treatment. Recent case reports have shown that there is also space for antihistamines in the treatment of LIRs. If symptoms remain after these treatment(s), hyaluronidase is still an effective treatment. Lastly, we still advise patients with soft tissue fillers to participate in any vaccination programs when needed.
Conclusions and perspectives
This follow-up review on LIRs after SARS-CoV-2 infection and vaccination shows that LIRs were still reported while the pandemic and vaccination programs continue. These adverse events were only reported after Moderna and Pfizer vaccines at the start of the vaccination programs. Although these mRNA vaccines remain the most common vaccines related to LIRs, they have also been reported for other types of vaccines such as AstraZeneca, Sputnik V, and Sinophram later on. Most of the reported LIRs are still self-limiting. We therefore still suggest that patients with soft tissue fillers continue to participate in vaccination programs when needed. However, medical staff should expand patients medical history and be aware of patients having soft tissue fillers.
Data availability
Complete data access is accessible through the corresponding author.
References
Ballin AC, Brandt FS, Cazzaniga A (2015) Dermal fillers: an update. Am J Clin Dermatol 16(4):271–283
Funt D, Pavicic T (2015) Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Plast Surg Nurs 35(1):13–32
Tezel A, Fredrickson GH (2008) The science of hyaluronic acid dermal fillers. J Cosmet Laser Ther 10(1):35–42
de Vries CG, Geertsma RE (2013) Clinical data on injectable tissue fillers: a review. Expert Rev Med Devices 10(6):835–853
Salwowska NM et al (2016) Physiochemical properties and application of hyaluronic acid: a systematic review. J Cosmet Dermatol 15(4):520–526
Broder KW, Cohen SR (2006) An overview of permanent and semipermanent fillers. Plast Reconstr Surg 118(3 Suppl):7S-14S
Owosho AA et al (2014) Orofacial dermal fillers: foreign body reactions, histopathologic features, and spectrometric studies. Oral Surg Oral Med Oral Pathol Oral Radiol 117(5):617–625
Christensen L et al (2013) Bacterial infection as a likely cause of adverse reactions to polyacrylamide hydrogel fillers in cosmetic surgery. Clin Infect Dis 56(10):1438–1444
Christensen L et al (2005) Adverse reactions to injectable soft tissue permanent fillers. Aesthetic Plast Surg 29(1):34–48
Kadouch JA et al (2013) Delayed-onset complications of facial soft tissue augmentation with permanent fillers in 85 patients. Dermatol Surg 39(10):1474–1485
Kadouch JA et al (2015) Granulomatous foreign-body reactions to permanent fillers: detection of CD123+ plasmacytoid dendritic cells. Am J Dermatopathol 37(2):107–114
Bachour Y, Kadouch JA, Niessen FB (2021) The aetiopathogenesis of late inflammatory reactions (LIRs) after soft tissue filler use: a systematic review of the literature. Aesthetic Plast Surg 45(4):1748–1759. https://doi.org/10.1007/s00266-021-02306-3
Marusza W et al (2019) Treatment of late bacterial infections resulting from soft-tissue filler injections. Infect Drug Resist 12:469–480
de Melo Carpaneda E, Carpaneda CA (2012) Adverse results with PMMA fillers. Aesthetic Plast Surg 36(4):955–63
Marusza W et al (2019) The impact of lifestyle upon the probability of late bacterial infection after soft-tissue filler augmentation. Infect Drug Resist 12:855–863
Alhede M et al (2014) Bacterial biofilm formation and treatment in soft tissue fillers. Pathog Dis 70(3):339–346
Wiest LG, Stolz W, Schroeder JA (2009) Electron microscopic documentation of late changes in permanent fillers and clinical management of granulomas in affected patients. Dermatol Surg 35(Suppl 2):1681–1688
Netsvyetayeva I et al (2018) Skin bacterial flora as a potential risk factor predisposing to late bacterial infection after cross-linked hyaluronic acid gel augmentation. Infect Drug Resist 11:213–222
Saththianathan M et al (2017) The role of bacterial biofilm in adverse soft-tissue filler reactions: a combined laboratory and clinical study. Plast Reconstr Surg 139(3):613–621
Micheels P (2001) Human anti-hyaluronic acid antibodies: is it possible? Dermatol Surg 27(2):185–191
Rudolph CM et al (1999) Foreign body granulomas due to injectable aesthetic microimplants. Am J Surg Pathol 23(1):113–117
Alijotas-Reig J, Fernandez-Figueras MT, Puig L (2013) Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers. Semin Arthritis Rheum 43(2):241–258
Alijotas-Reig J et al (2012) Autoimmune/inflammatory syndrome (ASIA) induced by biomaterials injection other than silicone medical grade. Lupus 21(12):1326–1334
Lombardi T et al (2004) Orofacial granulomas after injection of cosmetic fillers Histopathologic and clinical study of 11 cases. J Oral Pathol Med 33(2):115–20
Israeli E et al (2009) Adjuvants and autoimmunity. Lupus 18(13):1217–1225
Food and Drug Administration (2020) FDA briefing document Moderna COVID-19 vaccine. Available from: https://www.fda.gov/media/144434/download. Accessed June 2023
Munavalli GG et al (2021) COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res 314(1):1–15. https://doi.org/10.1007/s00403-021-02190-6
Munavalli GG et al (2021) Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination-a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep 10:63–68
Rowland-Warmann MJ (2021) Hypersensitivity reaction to hyaluronic acid dermal filler following novel coronavirus infection - a case report. J Cosmet Dermatol 20(5):1557–1562
Shome D et al (2021) Delayed hypersensitivity reaction to hyaluronic acid dermal filler post-COVID-19 viral infection. J Cosmet Dermatol 20(5):1549–1550
Naouri M et al (2021) Good tolerance of hyaluronic acid injections during the period of the COVID-19 pandemic: observing a cohort of 1093 patients in a prospective, observational real-life study. J Eur Acad Dermatol Venereol 35(7):e432–e433
McMahon DE et al (2021) Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol 85(1):46–55
Bachour Y et al (2022) Late inflammatory reactions in patients with soft tissue fillers after SARS-CoV-2 infection and vaccination: a systematic review of the literature. J Cosmet Dermatol 21(4):1361–1368
Fan Y et al (2022) SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct Target Ther 7(1):141
WHO (2024) Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic. Available from: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic. Accessed June 2023
Moher D et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–9
Nedicine LO (2009) Oxford centre for evidence-based medicine – levels of evidence. University of Oxford, Oxford
Liu L, Ledinh W (2022) COVID-19 infection-associated hypersensitivity reaction to dermal filler-a case report and review of the histologic features. J Cosmet Dermatol 21(9):3673–3674
Virdi G (2022) Dermal fillers and COVID-19: angioedema with urticaria in a patient post COVID-19 infection. Cureus 14(4):e24461
Kato K et al (2022) Increase in the incidence of acute inflammatory reactions to injectable fillers during COVID-19 era. J Cosmet Dermatol 21(5):1816–1821
Michon A (2021) Hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID-19 vaccination - a case report. J Cosmet Dermatol 20(9):2684–2690
Savva D et al (2021) Hypersensitivity reaction to hyaluronic acid dermal filler after the Pfizer vaccination against SARS-CoV-2. Int J Infect Dis 113:233–235
Osmond A, Kenny B (2021) Reaction to dermal filler following COVID-19 vaccination. J Cosmet Dermatol 20(12):3751–3752
Beamish IV, Bogoch II, Carr D (2022) Delayed inflammatory reaction to dermal fillers after COVID-19 vaccination: a case report. CJEM 24(4):444–446
Alharithy R, Alsaedi A, Alsaedi M (2022) Postmessenger ribonucleic acid COVID-19 vaccine delayed inflammatory reaction to dermal fillers: case report. J Dermatol Dermatol Surg-Jdds 26(1):48–50
Azzouz S et al (2023) Delayed hypersensitivity reaction to cosmetic filler following two COVID-19 vaccinations and infection. Allergy Asthma Clin Immunol 19(1):31
Kalantari Y et al (2022) First reported case of delayed-type hypersensitivity reaction to non-hyaluronic acid Polycaprolactone dermal filler following COVID-19 vaccination: a case report and a review of the literature. Clin Case Rep 10(2):e05343
Alijotas-Reig J et al (2022) Inflammatory immune-mediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol 21(8):3181–3187
Ortigosa LCM et al (2022) Hypersensitivity reaction to hyaluronic acid dermal filler after COVID-19 vaccination: a series of cases in Sao Paulo. Brazil Int J Infect Dis 116:268–270
Jeon HB, Yoon JH, Lim NK (2022) Midface infection after COVID-19 vaccination in a patient with calcium hydroxylapatite dermal filler: a case report and literature review. Arch Plast Surg 49(3):310–314
Decates T et al (2021) Immediate nor delayed type hypersensitivity plays a role in late inflammatory reactions after hyaluronic acid filler injections. Clin Cosmet Investig Dermatol 14:581–589
Decates TS et al (2021) Increased risk of late-onset, immune-mediated, adverse reactions related to dermal fillers in patients bearing HLA-B*08 and DRB1*03 haplotypes. Dermatol Ther 34(1):e14644
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by YB. The first draft of the manuscript was written by YB, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This is a systematic review; therefore, no ethical approval is required.
Consent to participate
This article does not contain any studies with human participants or animals performed by the author.
Consent for publication
This is a systematic review; therefore, no consent to publish is required.
Competing interests
Yara Bachour declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bachour, Y. An update and overview of the literature on late inflammatory reactions (LIRs) in soft tissue fillers after SARS-CoV-2 infection and vaccination. Eur J Plast Surg 46, 855–864 (2023). https://doi.org/10.1007/s00238-023-02121-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-023-02121-w