Abstract
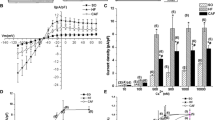
Recent studies have shown that the sensitivity of apamin-sensitive K+ current (I KAS, mediated by apamin-sensitive small conductance calcium-activated potassium channels subunits) to intracellular Ca2+ is increased in heart failure (HF), leading to I KAS upregulation, action potential duration shortening, early after depolarization, and recurrent spontaneous ventricular fibrillation. We hypothesized that casein kinase 2 (CK2) interacted with small conductance calcium-activated potassium channels (SK) is decreased in HF, and protein phosphatase 2A (PP2A) is increased on the opposite, upregulating the sensitivity of I KAS to intracellular Ca2+ in HF. Rat model of volume-overload HF was established by an abdominal arteriovenous fistula procedure. The expression of SK channels, PP2A and CK2 was detected by Western blot analysis. Interaction and colocalization of CK2 with SK channel were detected by co-immunoprecipitation analysis and double immunofluorescence staining. In HF rat left ventricle, SK3 was increased by 100 % (P < 0.05), and SK2 was not significantly changed. PP2A protein was increased by 94.7 % in HF rats (P < 0.05), whereas the level of CK2 was almost unchanged. We found that CK2 colocalized with SK2 and SK3 in rat left ventricle. With anti-CK2α antibody, SK2 and SK3 were immunoprecipitated, the level of precipitated SK2 decreased by half, whereas precipitated SK3 was almost unchanged. In conclusion, the increased expression of total PP2A and decreased interaction of CK2 with SK2 may underlie enhanced sensitivity of I KAS to intracellular Ca2+ in volume-overload HF rat.





Similar content being viewed by others
References
Allen D, Fakler B, Maylie J, Adelman JP (2007) Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J Neurosci 27:2369–2376. doi:10.1523/JNEUROSCI.3565-06.2007
Arai M, Matsui H, Periasamy M (1994) Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res 74:555–564. doi:10.1161/01.RES.74.4.555
Baartscheer A, Schumacher CA, Belterman CN, Coronel R, Fiolet JW (2003) [Na+]i and the driving force of the Na+/Ca2+-exchanger in heart failure. Cardiovasc Res 57:986–995. doi:10.1016/S0008-6363(02)00848-9
Bers DM (2008) Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70:23–49. doi:10.1146/annurev.physiol.70.113006.100455
Bers DM (2011) Ca2+-calmodulin-dependent protein kinase II regulation of cardiac excitation-transcription coupling. Heart Rhythm 8:1101–1104. doi:10.1016/j.hrthm.2011.01.030
Bildl W, Strassmaier T, Thurm H, Andersen J, Eble S, Oliver D, Knipper M, Mann M, Schulte U, Adelman JP, Fakler B (2004) Protein kinase CK2 is coassembled with small conductance Ca2+-activated K+ channels and regulates channel gating. Neuron 43:847–858. doi:10.1016/j.neuron.2004.08.033
Castle NA, Haylett DG, Jenkinson DH (1989) Toxins in the characterization of potassium channels. Trends Neurosci 12:59–65. doi:10.1016/0166-2236(89)90137-9
Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH (2009) Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest 119:1940–1951. doi:10.1172/JCI37059
Chua SK, Chang PC, Maruyama M, Turker I, Shinohara T, Shen MJ, Chen Z, Shen C, Rubart-von DLM, Lopshire JC, Ogawa M, Weiss JN, Lin SF, Ai T, Chen PS (2011) Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res 108:971–979. doi:10.1161/CIRCRESAHA.110.238386
DeGrande ST, Little SC, Nixon DJ, Wright P, Snyder J, Dun W, Murphy N, Kilic A, Higgins R, Binkley PF, Boyden PA, Carnes CA, Anderson ME, Hund TJ, Mohler PJ (2013) Molecular mechanisms underlying cardiac protein phosphatase 2A regulation in heart. J Biol Chem 288:1032–1046. doi:10.1074/jbc.M112.426957
Diness JG, Sorensen US, Nissen JD, Al-Shahib B, Jespersen T, Grunnet M, Hansen RS (2010) Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circ Arrhythm Electrophysiol 3:380–390. doi:10.1161/CIRCEP.110.957407
Diness JG, Skibsbye L, Jespersen T, Bartels ED, Sorensen US, Hansen RS, Grunnet M (2011) Effects on atrial fibrillation in aged hypertensive rats by Ca2+-activated K+ channel inhibition. Hypertension 57:1129–1135. doi:10.1161/HYPERTENSIONAHA.111.170613
Du Y, Huang X, Wang T, Han K, Zhang J, Xi Y, Wu G, Ma A (2007) Downregulation of neuronal sodium channel subunits Nav1.1 and Nav1.6 in the sinoatrial node from volume-overloaded heart failure rat. Pflugers Arch 454:451–459. doi:10.1007/s00424-007-0216-4
Ellinor PT, Lunetta KL, Glazer NL, Pfeufer A, Alonso A, Chung MK, Sinner MF, de Bakker PI, Mueller M, Lubitz SA, Fox E, Darbar D, Smith NL, Smith JD, Schnabel RB, Soliman EZ, Rice KM, Van Wagoner DR, Beckmann BM, van Noord C, Wang K, Ehret GB, Rotter JI, Hazen SL, Steinbeck G, Smith AV, Launer LJ, Harris TB, Makino S, Nelis M, Milan DJ, Perz S, Esko T, Kottgen A, Moebus S, Newton-Cheh C, Li M, Mohlenkamp S, Wang TJ, Kao WH, Vasan RS, Nothen MM, MacRae CA, Stricker BH, Hofman A, Uitterlinden AG, Levy D, Boerwinkle E, Metspalu A, Topol EJ, Chakravarti A, Gudnason V, Psaty BM, Roden DM, Meitinger T, Wichmann HE, Witteman JC, Barnard J, Arking DE, Benjamin EJ, Heckbert SR, Kaab S (2010) Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet 42:240–244. doi:10.1038/ng.537
Grimm D, Holmer SR, Riegger GA, Kromer EP (1999) Effects of beta-receptor blockade and angiotensin II type I receptor antagonism in isoproterenol-induced heart failure in the rat. Cardiovasc Pathol 8:315–323. doi:10.1016/S0008-6363(02)00848-9
Gui L, Bao Z, Jia Y, Qin X, Cheng ZJ, Zhu J, Chen QH (2013) Ventricular tachyarrhythmias in rats with acute myocardial infarction involves activation of small-conductance Ca2+-activated K + channels. Am J Physiol Heart Circ Physiol 304:H118–H130. doi:10.1152/ajpheart.00820.2011
He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, Anderson ME (2011) Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med 17:1610–1618. doi:10.1038/nm.2506
Hjalmarson A (1999) Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353:2001–2007. doi:10.1016/S0140-6736(99)04440-2
Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW (2009) 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 53:e1–e90. doi:10.1016/j.jacc.2008.11.013
Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q, Zhang Z, Singapuri A, Albert TR, Rajagopal AV, Bond CT, Periasamy M, Adelman J, Chiamvimonvat N (2009) Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol 587:1087–1100. doi:10.1113/jphysiol.2008.167718
Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA, Chiamvimonvat N (2007) Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res 100:112–120. doi:10.1161/01.RES.0000253095.44186.72
Lu L, Timofeyev V, Li N, Rafizadeh S, Singapuri A, Harris TR, Chiamvimonvat N (2009) Alpha-actinin2 cytoskeletal protein is required for the functional membrane localization of a Ca2+-activated K+ channel (SK2 channel). Proc Natl Acad Sci USA 106:18402–18407. doi:10.1073/pnas.0908207106
Marionneau C, Couette B, Liu J, Li H, Mangoni ME, Nargeot J, Lei M, Escande D, Demolombe S (2005) Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J Physiol 562:223–234. doi:10.1113/jphysiol.2004.074047
Michael G, Xiao L, Qi XY, Dobrev D, Nattel S (2009) Remodelling of cardiac repolarization: how homeostatic responses can lead to arrhythmogenesis. Cardiovasc Res 81:491–499. doi:10.1093/cvr/cvn266
Mulder P, Barbier S, Chagraoui A, Richard V, Henry JP, Lallemand F, Renet S, Lerebours G, Mahlberg-Gaudin F, Thuillez C (2004) Long-term heart rate reduction induced by the selective If current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation 109:1674–1679. doi:10.1161/01.CIR.0000118464.48959.1C
Nattel S (2009) Calcium-activated potassium current: a novel ion channel candidate in atrial fibrillation. J Physiol 587:1385–1386. doi:10.1113/jphysiol.2009.170621
Ni Y, Wang T, Zhuo X, Song B, Zhang J, Wei F, Bai H, Wang X, Yang D, Gao L, Ma A (2013) Bisoprolol reversed small conductance calcium-activated potassium channel (SK) remodeling in a volume-overload rat model. Mol Cell Biochem 384:95–103. doi:10.1007/s11010-013-1785-5
Ozgen N, Dun W, Sosunov EA, Anyukhovsky EP, Hirose M, Duffy HS, Boyden PA, Rosen MR (2007) Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc Res 75:758–769. doi:10.1016/j.cardiores.2007.05.008
Reiken S, Gaburjakova M, Gaburjakova J, He KK, Prieto A, Becker E, Yi GG, Wang J, Burkhoff D, Marks AR (2001) Beta-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation 104:2843–2848. doi:10.1161/hc4701.099578
Reiken S, Wehrens XH, Vest JA, Barbone A, Klotz S, Mancini D, Burkhoff D, Marks AR (2003) Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation 107:2459–2466. doi:10.1161/01.CIR.0000068316.53218.49
Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR (2010) Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest 120:4388–4398. doi:10.1172/JCI32726
Shelton RJ, Clark AL, Goode K, Rigby AS, Houghton T, Kaye GC, Cleland JG (2009) A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE-II Study). Heart 95:924–930. doi:10.1136/hrt.2008.158931
Skibsbye L, Diness JG, Sorensen US, Hansen RS, Grunnet M (2011) The duration of pacing-induced atrial fibrillation is reduced in vivo by inhibition of small conductance Ca2+-activated K+ channels. J Cardiovasc Pharmacol 57:672–681. doi:10.1097/FJC.0b013e318217943d
Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen PS, Efimov I, Dobrev D, Mohler PJ, Hund TJ, Anderson ME (2011) Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest 121:3277–3288. doi:10.1172/JCI57833
Terentyev D, Rochira JA, Terentyeva R, Roder K, Koren G, Li W (2014) Sarcoplasmic reticulum Ca(2)(+) release is both necessary and sufficient for SK channel activation in ventricular myocytes. Am J Physiol Heart Circ Physiol 306:H738–H746. doi:10.1152/ajpheart.00621.2013
Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, Xu Y, Nie L, Vazquez AE, Young JN, Glatter KA, Chiamvimonvat N (2005) Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol 289:H2714–H2723. doi:10.1152/ajpheart.00534.2005
Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, Nie L, Tuxson HR, Young JN, Glatter KA, Vazquez AE, Yamoah EN, Chiamvimonvat N (2003) Molecular identification and functional roles of a Ca2+-activated K+ channel in human and mouse hearts. J Biol Chem 278:49085–49094. doi:10.1074/jbc.M307508200
Yu T, Deng C, Wu R, Guo H, Zheng S, Yu X, Shan Z, Kuang S, Lin Q (2012) Decreased expression of small-conductance Ca2+-activated K+ channels SK1 and SK2 in human chronic atrial fibrillation. Life Sci 90:219–227. doi:10.1016/j.lfs.2011.11.008
Zhang Q, Timofeyev V, Lu L, Li N, Singapuri A, Long MK, Bond CT, Adelman JP, Chiamvimonvat N (2008) Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circ Res 102:465–471. doi:10.1161/CIRCRESAHA.107.161778
Acknowledgments
We thank Lin Yang, Juan Zhou, and Tao Geng for their help in the experimental technique. This study was supported by the State Key Program of the National Natural Science Foundation of China (NSFC, No. 30830051) and the Science and Technology Program for Public Wellbeing (No: 2012GS610101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ehical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yang, D., Wang, T., Ni, Y. et al. Apamin-Sensitive K+ Current Upregulation in Volume-Overload Heart Failure is Associated with the Decreased Interaction of CK2 with SK2. J Membrane Biol 248, 1181–1189 (2015). https://doi.org/10.1007/s00232-015-9839-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-015-9839-0