Abstract
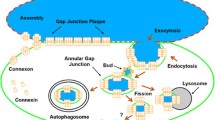
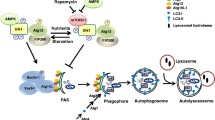
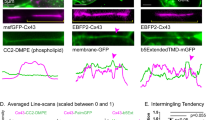
Gap junctions (GJs) are composed of tens to many thousands of double-membrane spanning GJ channels that cluster together to form densely packed channel arrays (termed GJ plaques) in apposing plasma membranes of neighboring cells. In addition to providing direct intercellular communication (GJIC, their hallmark function), GJs, based on their characteristic double-membrane-spanning configuration, likely also significantly contribute to physical cell-to-cell adhesion. Clearly, modulation (up-/down-regulation) of GJIC and of physical cell-to-cell adhesion is as vitally important as the basic ability of GJ formation itself. Others and we have previously described that GJs can be removed from the plasma membrane via the internalization of entire GJ plaques (or portions thereof) in a cellular process that resembles clathrin-mediated endocytosis. GJ endocytosis results in the formation of double-membrane vesicles [termed annular gap junctions (AGJs) or connexosomes] in the cytoplasm of one of the coupled cells. Four recent independent studies, consistent with earlier ultrastructural analyses, demonstrate the degradation of endocytosed AGJ vesicles via autophagy. However, in TPA-treated cells others report degradation of AGJs via the endo-/lysosomal degradation pathway. Here we summarize evidence that supports the concept that autophagy serves as the cellular default pathway for the degradation of internalized GJs. Furthermore, we highlight and discuss structural criteria that seem required for an alternate degradation via the endo-/lysosomal pathway.



Similar content being viewed by others
Abbreviations
- AGJ:
-
Annular gap junction
- CME:
-
Clathrin mediated endocytosis
- Cx:
-
Connexin
- DAG:
-
Diacylglycerol
- GFP:
-
Green fluorescent protein
- GJ:
-
Gap junction
- GJIC:
-
Gap junction mediated intercellular communication
- LAMP:
-
Lysosomal associated membrane protein
- LC3:
-
Microtubule-associated protein light chain 3
- PAEC:
-
Pulmonary artery endothelial cell
- PE:
-
Phosphatidyl-ethanolamine
- PKC:
-
Protein kinase C
- RNAi:
-
RNA interference
- SQSTM1:
-
Sequestosome 1
- SUMO:
-
Small Ub-like modifier
- TPA:
-
12-O-tetradecanoylphorbol 13-acetate
- Ub:
-
Ubiquitin
- WGA:
-
Wheat germ agglutinin
- YFP:
-
Yellow fluorescent protein
References
Baker SM, Kim N, Gumpert AM, Segretain D, Falk MM (2008) Acute internalization of gap junctions in vascular endothelial cells in response to inflammatory mediator-induced G-protein coupled receptor activation. FEBS Lett 582:4039–4046
Baldwin AL, Thurston G (2001) Mechanics of endothelial cell architecture and vascular permeability. Crit Rev Biomed Eng 29:247–278
Barriere H, Nemes C, Lechardeur D, Khan-Mohammad M, Fruh K, Lukacs GL (2006) Molecular basis of oligoubiquitin-dependent internalization of membrane proteins in Mammalian cells. Traffic 7:282–297
Beardslee MA, Laing JG, Beyer EC, Saffitz JE (1998) Rapid turnover of connexin43 in the adult rat heart. Circ Res 83:629–635
Bejarano E, Girao H, Yuste A, Patel B, Marques C, Spray DC, Pereira P, Cuervo AM (2012) Autophagy modulates dynamics of connexins at the plasma membrane in a ubiquitin-dependent manner. Mol Biol Cell 23:2156–2169
Belouzard S, Rouille Y (2006) Ubiquitylation of leptin receptor OB-Ra regulates its clathrin-mediated endocytosis. EMBO J 25:932–942
Berthoud VM, Minogue PJ, Laing JG, Beyer EC (2004) Pathways for degradation of connexins and gap junctions. Cardiovasc Res 62:256–267
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614
Blomstrand F, Venance L, Siren AL, Ezan P, Hanse E, Glowinski J, Ehrenreich H, Giaume C (2004) Endothelins regulate astrocyte gap junctions in rat hippocampal slices. Eur J Neurosci 19:1005–1015
Catarino S, Ramalho JS, Marques C, Pereira P, Girao H (2011) Ubiquitin-mediated internalization of connexin43 is independent of the canonical endocytic tyrosine-sorting signal. Biochem J 437:255–267
Ciani B, Layfield R, Cavey JR, Sheppard PW, Searle MS (2003) Structure of the ubiquitin-associated domain of p62 (SQSTM1) and implications for mutations that cause Paget’s disease of bone. J Biol Chem 278:37409–37412
Delmar M, Coombs W, Sorgen P, Duffy HS, Taffet SM (2004) Structural bases for the chemical regulation of connexin43 channels. Cardiovasc Res 62:268–275
Falk MM (2010) Adherens junctions remain dynamic. BMC Biol 8:34
Falk MM, Baker SM, Gumpert AM, Segretain D, Buckheit RW 3rd (2009) Gap junction turnover is achieved by the internalization of small endocytic double-membrane vesicles. Mol Biol Cell 20:3342–3352
Fallon RF, Goodenough DA (1981) Five-hour half-life of mouse liver gap-junction protein. J Cell Biol 90:521–526
Fong JT, Kells RM, Gumpert AM, Marzillier JY, Davidson MW, Falk MM (2012) Internalized gap junctions are degraded by autophagy. Autophagy 8:794–811
Fushman D, Wilkinson KD (2011) Structure and recognition of polyubiquitin chains of different lengths and linkage. F1000 Biol Rep 3:26
Fykerud TA, Kjenseth A, Schink KO, Sirnes S, Bruun J, Omori Y, Brech A, Rivedal E, Leithe E (2012) Smad ubiquitination regulatory factor-2 controls gap junction intercellular communication by modulating endocytosis and degradation of connexin43. J Cell Sci. doi:10.1242/j cs.093500
Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH (2002) Multicolor and electron microscopic imaging of connexin trafficking. Science 296:503–507
Geetha T, Jiang J, Wooten MW (2005) Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol Cell 20:301–312
Ghoshroy S, Goodenough DA, Sosinsky GE (1995) Preparation, characterization, and structure of half gap junctional layers split with urea and EGTA. J Membr Biol 146:15–28
Giepmans BN, Moolenaar WH (1998) The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol 8:931–934
Gilleron J, Fiorini C, Carette D, Avondet C, Falk MM, Segretain D, Pointis G (2008) Molecular reorganization of Cx43, Zo-1 and Src complexes during the endocytosis of gap junction plaques in response to a non-genomic carcinogen. J Cell Sci 121:4069–4078
Ginzberg RD, Gilula NB (1979) Modulation of cell junctions during differentiation of the chicken otocyst sensory epithelium. Dev Biol 68:110–129
Girao H, Catarino S, Pereira P (2009) Eps15 interacts with ubiquitinated Cx43 and mediates its internalization. Exp Cell Res 315:3587–3597
Goodenough DA, Gilula NB (1974) The splitting of hepatocyte gap junctions and zonulae occludentes with hypertonic disaccharides. J Cell Biol 61:575–590
Gumpert AM, Varco JS, Baker SM, Piehl M, Falk MM (2008) Double-membrane gap junction internalization requires the clathrin-mediated endocytic machinery. FEBS Lett 582:2887–2892
Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J (2010) Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 141:656–667
Hawryluk MJ, Keyel PA, Mishra SK, Watkins SC, Heuser JE, Traub LM (2006) Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic 7:262–281
Hesketh GG, Shah MH, Halperin VL, Cooke CA, Akar FG, Yen TE, Kass DA, Machamer CE, Van Eyk JE, Tomaselli GF (2010) Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res 106:1153–1163
Hicke L (2001) Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol 2:195–201
Hicke L, Dunn R (2003) Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol 19:141–172
Hung SY, Huang WP, Liou HC, Fu WM (2009) Autophagy protects neuron from Abeta-induced cytotoxicity. Autophagy 5:502–510
Hunter AW, Barker RJ, Zhu C, Gourdie RG (2005) Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell 16:5686–5698
Jordan K, Chodock R, Hand AR, Laird DW (2001) The origin of annular junctions: a mechanism of gap junction internalization. J Cell Sci 114:763–773
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Kjenseth A, Fykerud T, Rivedal E, Leithe E (2010) Regulation of gap junction intercellular communication by the ubiquitin system. Cell Signal 22:1267–1273
Kjenseth A, Fykerud TA, Sirnes S, Bruun J, Kolberg M, Yohannes Z, Omori Y, Rivedal E, Leithe E (2012) The gap junction channel protein connexin43 is covalently modified and regulated by SUMOylation. J Biol Chem 287:15851–15861
Laing JG, Tadros PN, Westphale EM, Beyer EC (1997) Degradation of connexin43 gap junctions involves both the proteasome and the lysosome. Exp Cell Res 236:482–492
Laird DW (2005) Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta 1711:172–182
Lampe PD, Lau AF (2004) The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 36:1171–1186
Lane NJ, Swales LS (1980) Dispersal of junctional particles, not internalization, during the in vivo disappearance of gap junctions. Cell 19:579–586
Larsen WJ, Tung HN, Murray SA, Swenson CA (1979) Evidence for the participation of actin microfilaments and bristle coats in the internalization of gap junction membrane. J Cell Biol 83:576–587
Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM (2002) Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci USA 99:10446–10451
Leach DH, Oliphant LW (1984) Degradation of annular gap junctions of the equine hoof wall. Acta Anat (Basel) 120:214–219
Leithe E, Rivedal E (2004a) Epidermal growth factor regulates ubiquitination, internalization and proteasome-dependent degradation of connexin43. J Cell Sci 117:1211–1220
Leithe E, Rivedal E (2004b) Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-O-tetradecanoylphorbol 13-acetate treatment. J Biol Chem 279:50089–50096
Leithe E, Kjenseth A, Sirnes S, Stenmark H, Brech A, Rivedal E (2009) Ubiquitylation of the gap junction protein connexin-43 signals its trafficking from early endosomes to lysosomes in a process mediated by Hrs and Tsg101. J Cell Sci 122:3883–3893
Leithe E, Sirnes S, Fykerud T, Kjenseth A, Rivedal E (2011) Endocytosis and post-endocytic sorting of connexins. Biochim Biophys Acta 1818:1870–1879
Lichtenstein A, Minogue PJ, Beyer EC, Berthoud VM (2011) Autophagy: a pathway that contributes to connexin degradation. J Cell Sci 124:910–920
Lum H, Malik AB (1994) Regulation of vascular endothelial barrier function. Am J Physiol 267:L223–L241
Madshus IH (2006) Ubiquitin binding in endocytosis—how tight should it be and where does it happen? Traffic 7:258–261
Mari M, Tooze SA, Reggiori F (2011) The puzzling origin of the autophagosomal membrane. F1000 Biol Rep 3:25
Matsunaga K, Morita E, Saitoh T, Akira S, Ktistakis NT, Izumi T, Noda T, Yoshimori T (2010) Autophagy requires endoplasmic reticulum targeting of the PI3-kinase complex via Atg14L. J Cell Biol 190:511–521
Mazet F, Wittenberg BA, Spray DC (1985) Fate of intercellular junctions in isolated adult rat cardiac cells. Circ Res 56:195–204
Mizushima N (2004) Methods for monitoring autophagy. Int J Biochem Cell Biol 36:2491–2502
Moreno AP (2005) Connexin phosphorylation as a regulatory event linked to channel gating. Biochim Biophys Acta 1711:164–171
Musil LS, Le AC, VanSlyke JK, Roberts LM (2000) Regulation of connexin degradation as a mechanism to increase gap junction assembly and function. J Biol Chem 275:25207–25215
Pahujaa M, Anikin M, Goldberg GS (2007) Phosphorylation of connexin43 induced by Src: regulation of gap junctional communication between transformed cells. Exp Cell Res 313:4083–4090
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
Pfeifer U (1980) Autophagic sequestration of internalized gap junctions in rat liver. Eur J Cell Biol 21:244–246
Piehl M, Lehmann C, Gumpert A, Denizot JP, Segretain D, Falk MM (2007) Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol Biol Cell 18:337–347
Pohl C, Jentsch S (2009) Midbody ring disposal by autophagy is a post-abscission event of cytokinesis. Nat Cell Biol 11:65–70
Postma FR, Hengeveld T, Alblas J, Giepmans BN, Zondag GC, Jalink K, Moolenaar WH (1998) Acute loss of cell–cell communication caused by G protein-coupled receptors: a critical role for c-Src. J Cell Biol 140:1199–1209
Qin H, Shao Q, Igdoura SA, Alaoui-Jamali MA, Laird DW (2003) Lysosomal and proteasomal degradation play distinct roles in the life cycle of Cx43 in gap junctional intercellular communication-deficient and -competent breast tumor cells. J Biol Chem 278:30005–30014
Ravikumar B, Imarisio S, Sarkar S, O’Kane CJ, Rubinsztein DC (2008) Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J Cell Sci 121:1649–1660
Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC (2010) Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol 12:747–757
Rhett JM, Gourdie RG (2011) The perinexus: a new feature of Cx43 gap junction organization. Heart Rhythm 9:619–623
Rhett JM, Jourdan J, Gourdie RG (2011) Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell 22:1516–1528
Schnell DJ, Hebert DN (2003) Protein translocons: multifunctional mediators of protein translocation across membranes. Cell 112:491–505
Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW (2004) Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol 24:8055–8068
Severs NJ, Shovel KS, Slade AM, Powell T, Twist VW, Green CR (1989) Fate of gap junctions in isolated adult mammalian cardiomyocytes. Circ Res 65:22–42
Shih SC, Katzmann DJ, Schnell JD, Sutanto M, Emr SD, Hicke L (2002) Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat Cell Biol 4:389–393
Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S (2005) Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA 102:2760–2765
Solan JL, Lampe PD (2009) Connexin43 phosphorylation: structural changes and biological effects. Biochem J 419:261–272
Spinella F, Rosano L, Di Castro V, Nicotra MR, Natali PG, Bagnato A (2003) Endothelin-1 decreases gap junctional intercellular communication by inducing phosphorylation of connexin 43 in human ovarian carcinoma cells. J Biol Chem 278:41294–41301
Stahl PD, Barbieri MA (2002) Multivesicular bodies and multivesicular endosomes: the “ins and outs” of endosomal traffic. Sci STKE 2002:pe32
Su V, Lau AF (2012) Ubiquitination, intracellular trafficking, and degradation of connexins. Arch Biochem Biophys 524:16–22
Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M (1998) Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 273:12725–12731
van Zeijl L, Ponsioen B, Giepmans BN, Ariaens A, Postma FR, Varnai P, Balla T, Divecha N, Jalink K, Moolenaar WH (2007) Regulation of connexin43 gap junctional communication by phosphatidylinositol 4,5-bisphosphate. J Cell Biol 177:881–891
Warn-Cramer BJ, Lau AF (2004) Regulation of gap junctions by tyrosine protein kinases. Biochim Biophys Acta 1662:81–95
Wilkinson CR, Seeger M, Hartmann-Petersen R, Stone M, Wallace M, Semple C, Gordon C (2001) Proteins containing the UBA domain are able to bind to multi-ubiquitin chains. Nat Cell Biol 3:939–943
Wong CW, Christen T, Kwak BR (2004) Connexins in leukocytes: shuttling messages? Cardiovasc Res 62:357–367
Wright CS (1984) Structural comparison of the two distinct sugar binding sites in wheat germ agglutinin isolectin II. J Mol Biol 178:91–104
Yen WL, Shintani T, Nair U, Cao Y, Richardson BC, Li Z, Hughson FM, Baba M, Klionsky DJ (2010) The conserved oligomeric Golgi complex is involved in double-membrane vesicle formation during autophagy. J Cell Biol 188:101–114
Acknowledgments
Work in the laboratory of M.M.F. is supported by NIHs NIGMS (grant GM55725) and by Lehigh University. We thank additional members of the Falk laboratory for critical comments. We wish to extend our sincere appreciation to Ross Johnson, whose dedication to gap junctions has revealed so many exciting aspects of this truly amazing cellular structure. His excitement and passion has fueled this research and will fuel the passion of many generations of researchers to come. Although we know so much about gap junctions, so much more still needs to be discovered! His contributions to this field have been significant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Falk, M.M., Fong, J.T., Kells, R.M. et al. Degradation of Endocytosed Gap Junctions by Autophagosomal and Endo-/lysosomal Pathways: A Perspective. J Membrane Biol 245, 465–476 (2012). https://doi.org/10.1007/s00232-012-9464-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-012-9464-0