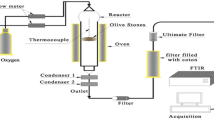
Abstract
The main purpose of this work is to identify the hygroscopic equilibrium and also determine the product–water relationship in olive pomace. In this context, experimental, modeling and thermodynamic analysis of adsorption isotherms of four types of agricultural wastes (olive pomace), have been carried out. Two samples were separately de-oiled by the maceration method and the hydrothermal carbonization process. Besides, adsorption isotherms of these biomass samples were obtained experimentally at three oven temperatures (30, 40 and 50 °C). The findings showed that the de-oiled samples are more hydrophobic than those containing residual oil, especially when water activity (aw) values are higher than 0.4. Moreover, adsorption isotherms were modeled by five mathematical models available in the literature. In the thermodynamic point of view, the net isosteric heat and the differential entropy of adsorption were estimated for each sample. The highest value of the net isosteric heat (around 65 kJ mol−1) is obtained for no de-oiled samples. Also, it has been found that the development of monolayer adsorption requires more net isosteric heat to ensure the water molecules adhesion to the biomass surface unlike the formation of multilayer adsorption. In addition, the enthalpy/entropy compensation theory for all examined biomasses was confirmed, and their free energy values are positive (between 3479.8 and 4155.6 J mol−1). Finally, optimal water activity values were also estimated and ranged from 0.35 to 0.49.







Similar content being viewed by others
Abbreviations
- A, B, C, D :
-
Model parameters (–)
- a w :
-
Water activity (–)
- d :
-
Number of degrees of freedom (–)
- HR :
-
Relative humidity (%)
- L v :
-
Latent heat of vaporization (J kg−1)
- M h :
-
Wet mass (kg)
- MRE:
-
Mean relative error (–)
- Ms :
-
Dry mass (kg)
- N :
-
Number of experimental points (–)
- n:
-
Number of variables of each model (–)
- q st :
-
Net isosteric heat of adsorption (J mol−1)
- Q st :
-
Total isosteric heat of adsorption (J kg−1)
- r :
-
Correlation coefficient (–)
- R:
-
Perfect gas constant (J mol−1 K−1)
- SEE:
-
Standard error of estimation (–)
- T:
-
Absolute temperature (K)
- X eq :
-
Equilibrium water content (kg water/kg d.b)
- \(\ell\) :
-
Number of isotherms (–)
- θ:
-
Temperature (°C)
- ΔG :
-
Gibbs free energy (J mol−1)
- ΔS:
-
Differential entropy (J mol−1 K−1)
- exp :
-
Experimental
- pre :
-
Predicted
- op :
-
Optimal
- hm :
-
Harmonic
- β :
-
Isokinetic
References
Elorf A, Sarh B, Tabet F, Bostyn S, Asbik M, Bonnamy S, Chaoufi J, Boushaki T, Gillon P (2019) Effect of swirl strength on the flow and combustion characteristics of pulverized biomass flames. Combust Sci Technol 191(4):629–644. https://doi.org/10.1080/00102202.2018.1497611
Bakhattar I, Bennini MA, Asbik M, Elorf A, Sarh B, Boushaki T (2018) CFD modeling of biomass (Olive Pomace) combustion in a fixed bed. In: Proceedings of the 6th International renewable and sustainable energy conference (IRSEC), IEEE, 2018. https://doi.org/10.1109/IRSEC.2018.8702277
Elorf A, Bakhattar I, Asbik M, Gillon P (2019) Fixed-bed biomass combustor: air mass flow rate and particles size effects on ignition front propagation of solid olive waste. J Combust Sci Technol. https://doi.org/10.1080/00102202.2019.1680070
Hepbasli A, Akdeniz RC, Vardar-Sukan F, Oktay Z (2003) Utilization of olive cake as a potential energy source in Turkey. Energy Sources 25:405–417. https://doi.org/10.1080/00908310303448
Miranda T, Arranz J, Montero I, Román S, Rojas C, Nogales S (2012) Characterization and combustion of olive pomace and forest residue pellets. Fuel Process Technol 103:91–96. https://doi.org/10.1016/j.fuproc.2011.10.016
Guizani C, Haddad K, Jeguirim M, Colin B, Limousy L (2016) Combustion characteristics and kinetics of torrefied olive pomace. Energy 107:453–463. https://doi.org/10.1016/j.energy.2016.04.034
Bennini MA, Koukouch A, Bakhattar I, Asbik M, Boushaki T, Sarh B, Elorf A, Cagnon B, Bonnamy S (2019) Characterization and combustion of olive pomace in a fixed bed boiler: effects of particle sizes. Int. J. Heat Technol. 37:229–238. https://doi.org/10.18280/ijht.370128
Koukouch A, Bakhattar I, Asbik M, Idlimam A, Zeghmati B, Aharoune A (2020) Analytical solution of coupled heat and mass transfer equations during convective drying of biomass: experimental validation. Heat Mass Transfer 56(377):1–13. https://doi.org/10.1007/s00231-020-02817-w
Koukouch A, Idlimam A, Asbik M, Sarh B, Izrar B, Bostyn S, Bah A, Ansari O, Zegaoui O, Amine A (2017) Experimental determination of the effective moisture diffusivity and activation energy during convective solar drying of olive pomace waste. Renew Energy 101:565–574. https://doi.org/10.1016/j.renene.2016.09.006
Nkolo Meze’e YN, Ngamveng JN, Bardet S (2008) Effect of enthalpy–entropy compensation during sorption of water vapour in tropical woods: The case of Bubinga (Guibourtia Tessmanii J. Leonard; G. Pellegriniana J.L.). Thermochim Acta 468:1–5. https://doi.org/10.1016/j.tca.2007.11.002
Ouertani S, Azzouz S, Hassini L, Koubaa A, Belghith A (2014) Moisture sorption isotherms and thermodynamic properties of Jack pine and palm wood: comparative study. Ind Crops Prod 56:200–210. https://doi.org/10.1016/j.indcrop.2014.03.004
Bahar R, Azzouz S, Remond R, Ouertani S, Elaieb MT, El Cafci MA (2016) Moisture sorption isotherms and thermodynamic properties of Oak wood (Quercus Robur and Quercus Canariensis): optimization of the processing parameters. Heat Mass Transfer 53(5):1541–1552. https://doi.org/10.1007/s00231-016-1916-0
Jiang X, Li H, Ramaswamy HS, Zhu S, Yu Y (2019) Moisture sorption isotherms and isosteric heats of sorption of high pressure treated paulownia wood under different storage conditions. Trans ASABE 62(1):105–114. https://doi.org/10.13031/trans.12899
Koukouch A, Idlimam A, Asbik M, Sarh B, Izrar B, Bah A, Ansari O (2015) Thermophysical characterization and mathematical modeling of convective solar drying of raw olive pomace characterization and mathematical modeling. Energy Convers Manage 99:221–230. https://doi.org/10.1016/j.enconman.2015.04.044
Vega-Gálvez A, Miranda M, Puente Díaz L, Lopez L, Rodriguez K, Di Scala K (2010) Effective moisture diffusivity determination and mathematical modeling of the drying curves of the olive-waste cake. Bioresour Technol 101:7265–7270. https://doi.org/10.1016/j.biortech.2010.04.040
Liébanes MD, Aragón JM, Palancar MC, Arévalo G, Jiménez D (2006) Equilibrium moisture isotherms of two-phase solid olive oil by-products: adsorption process thermodynamics. Colloids Surf Physicochem Eng Asp 282–283:298–306. https://doi.org/10.1016/j.colsurfa.2006.03.025
Liébanes MD, Aragón JM, Palancar MC (2008) Modeling the moisture sorption isotherms of two-phase solid olive oil by-product. Eur J Lipid Sci Technol 110:412–421. https://doi.org/10.1002/ejlt.200700230
Midilli A, Kucuk H, Yapar Z (2002) A new model for single layer drying. Drying Technol 20(7):1503–1513. https://doi.org/10.1081/DRT-120005864
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87(9–10):1051–1069. https://doi.org/10.1515/pac-2014-1117
Doymaz I, Gorel O, Akgun NA (2004) Drying characteristics of the solid by product of olive oil extraction. Biosyst Eng 88:213–219. https://doi.org/10.1016/j.biosystemseng.2004.03.003
Akgun NA, Doymaz I (2005) Modelling of olive cake thin-layer drying process. J Food Eng 68:455–461. https://doi.org/10.1016/j.jfoodeng.2004.06.023
Vlyssides AG, Loizides M, Karlis PK (2004) Integrated strategic approach for reusing olive oil extraction by-products. J Cleaner Prod 12:603–611. https://doi.org/10.1016/S0959-6526(03)00078-7
Missaoui A, Bostyna S, Belandria V, Cagnon B, Sarh B, Gökalp I (2017) Hydrothermal carbonization of dried olive pomace: energy potential and process performances. J Anal Appl Pyrolysis 128:281–290. https://doi.org/10.1016/j.jaap.2017.09.022
Alvarez-Murillo A, Sabio E, Ledesma B, Roman S, Gonzalez-García CM (2016) Generation of biofuel from hydrothermal carbonization of cellulose. Kinetics modelling. Energy 94:600–608. https://doi.org/10.1016/j.energy.2015.11.024
Naji A, Idlimam A, Kouhila M (2010) Sorption isotherms and thermodynamic properties of powdered milk. Chem Eng Commun 197:1109–1125. https://doi.org/10.1080/00986440903412936
Greenspan L (1977) Humidity fixed points of binary saturated aqueous solutions. J Res Nat Bur Stand 81(1):92–93. https://doi.org/10.6028/jres.081A.011
Menkov ND (2000) Moisture sorption isotherms of lentil seeds at several temperatures. J Food Eng 44(4):205–211. https://doi.org/10.1016/S0260-8774(00)00028-5
Wolf W, Spiess WEL, Jung G (1985). Standardization of isotherm measurements (Cost-Project 90 and 90 BIS). In: Simatos D, Multon JL (eds). Properties of water in foods. NATO ASI Series (Series E: Applied Sciences), 90:661–670. https://link.springer.com/chapter/10.1007%2F978-94-009-5103-7_40
Lahnine L, Idlimam A, Mahrouz M, Jada A, Hanine H, Mouhib M, Zantar S, Kouhila M (2016) Adsorption measurements and modeling of thyme treated with gamma irradiation and thermal-biochemical treatment. Ind Crops Prod 88:36–43. https://doi.org/10.1016/j.indcrop.2016.02.049
Bougayr EH, Lakhal EK, Idlimam A, Lamharrar A, Kouhila M, Berroug F (2018) Experimental study of hygroscopic equilibrium and thermodynamic properties of sewage sludge. Appl Therm Eng 143:521–531. https://doi.org/10.1016/j.applthermaleng.2018.07.048
Bernstein A, Noreña CPZ (2015) Thermodynamic sorption of red cabbage extract (Brassica oleracea L. var. capitata L. f. rubra) encapsulated by spray drying. J Food Sci Technol 52(12):8180–8187. https://doi.org/10.1007/s13197-015-1902-4
Gu Z, Yang J, Tao L, Liu F, Zhang Y (2021) Mathematical modelling of water sorption isotherms and thermodynamic properties of wastewater sewage sludge. Int J Low-Carbon Technol. https://doi.org/10.1093/ijlct/ctab029
Avramidis S (1992) Enthalpy-entropy compensation and thermodynamic considerations in sorption phenomena. Wood Sci Technol 26:329–333. https://doi.org/10.1007/BF00226074
Dalgıç AC, Pekmez H, Belibağlı KB (2012) Effect of drying methods on the moisture sorption isotherms and thermodynamic properties of mint leaves. J Food Sci Technol 49(4):439–449. https://doi.org/10.1007/s13197-011-0302-7
Beristain CI, Garcia HS, Azuara E (1996) Enthalpy-entropy compensation in food vapor adsorption. J Food Eng 30:405–415. https://doi.org/10.1016/S0260-8774(96)00011-8
Hao F, Lu L, Ge C (2014) Effect of fat content on water sorption properties of biscuits studied by nuclear magnetic resonance. J Food Nutr RES 2(11):814–818. https://doi.org/10.12691/jfnr-2-11-9
Roca E, Guillard V, Guilbert S, Gontard N (2006) Moisture migration in a cereal composite food at high water activity: effects of initial porosity and fat content. J Cereal Sci 43:144–151. https://doi.org/10.1016/j.jcs.2005.08.008
Marsh D (2013) Handbook of lipid bilayers, 2nd edn. CRC Press, New York
Liébanes MD, Aragón JM, Palancar MC, Arévalo G, Jiménez D (2006) Fluidized bed drying of 2-phase olive oil mill by-products. Drying Technol 24:1609–1618. https://doi.org/10.1080/07373930601031059
Sahasrabudhe SN, Rodriguez-Martinez V, O’Meara M, Farkas BE (2017) Density, viscosity, and surface tension of five vegetable oils at elevated temperatures: measurement and modeling. Int J Food Prop 20(2):1965–1981. https://doi.org/10.1080/10942912.2017.1360905
Lamharrar A, Idlimam A, Kouhila M, Lahnine L, Mouhanni H (2016) Moisture sorption isotherms and thermodynamic properties of Urtica Dioica leaves. Eur Sci J 12(24):376–388
Srikiatden J, Roberts JS (2006) Measuring moisture diffusivity of potato and carrot (core and cortex) during convective hot air and isothermal drying. J Food Eng 74:143–152. https://doi.org/10.1016/j.jfoodeng.2005.02.026
Acknowledgements
This work is supported by the research institute ‘’Institut de Recherche en Energie Solaire et Energies Nouvelles’’ via the BioF2S project funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
As announced in Section 5.2, this Appendix is only intended for the modeling results. To choose models adjusted to experimental adsorption isotherms, parameters A, B, C and D of the five models presented in Table 2 (see Section 3) should be determined. For this reason, the mean relative error (MRE) and the standard error of estimation (SEE) are simultaneously minimized and the correlation coefficient r is maximized too. So, in Tables
7,
8,
9, and
10, the (MRE) and (SEE) errors as well as the correlation coefficient r of the five models are compared with each other. Consequently, results from these tables confirm the good adequacy of three models (GAB, LESPAM and Peleg) with experimental results.
To identify the best model among them, residuals between experimental and predicted values (see Eq. (11)) for each biomass, are depicted in Figs. 8, 9, 10 and11. They show that the Peleg model describes perfectly adsorption isotherms of VOP biomass, and the LESPAM model is more suitable for adsorption isotherms of DOP_M. As for the GAB, it is the best adapted model to the two other samples (DOP_30 and DOP_HTC).
For these models, the mean relative error (MRE) and the correlation coefficient r have the lowest value and the highest value respectively for all the isotherms, and even more since their range of validity is appropriate to that of water activity.
Rights and permissions
About this article
Cite this article
Bakhattar, I., Koukouch, A., Chater, H. et al. Thermodynamic analysis of batch adsorption isotherms of different types of olive pomace. Heat Mass Transfer 58, 613–630 (2022). https://doi.org/10.1007/s00231-021-03120-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-021-03120-y