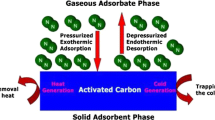
Abstract
Adsorption characteristics of nitrogen onto granular activated carbon for the wide range of temperature (303–323 K) and pressure (0.2027–2.0265 MPa) have been reported for a single bed pressure swing adsorption refrigeration system. The experimental data were fitted to Langmuir, Dubinin-Astakhov and Dubinin-Radushkevich (D-R) isotherms. The Langmuir and D-R isotherm models were found appropriate in correlating experimental adsorption data with an average relative error of ±2.0541% and ±0.6659% respectively. The isosteric heat of adsorption data were estimated as a function of surface coverage of nitrogen and temperature using D-R isotherm. The heat of adsorption was observed to decrease from 12.65 to 6.98 kJ.mol−1 with an increase in surface concentration at 303 K and it followed the same pattern for other temperatures. It was found that an increase in temperature enhances the magnitude of the heat of adsorption.










Similar content being viewed by others
Abbreviations
- A:
-
Van der Waals constant in Pa·m6.kmol−2
- ARE:
-
Average relative error in percentage
- B:
-
Van der Waals constant in m3.kmol−1
- b:
-
Measure of affinity of the nitrogen in the activated carbon in MPa−1
- b0 :
-
Pre-exponential factor in MPa−1
- dq:
-
The differential change in energy
- E:
-
Characteristic energy in kJ.mol−1
- Hads :
-
Heat of adsorption in kJ.mol−1
- Ms. :
-
The mass of adsorbent (activated carbon) in kg
- m:
-
The structural parameter of carbon
- N:
-
Number of data points
- n:
-
Number of moles of nitrogen adsorbed in mol.kg−1
- n0 :
-
Maximum monolayer surface adsorption capacity of activated carbon in mol.kg−1.
- n* :
-
The surface energy heterogeneity factor
- ncal :
-
Calculated number of moles of nitrogen adsorbed in mol.kg−1
- nexp :
-
Experimental number of moles of nitrogen adsorbed in mol.kg−1
- P:
-
Pressure in MPa
- Pc :
-
Critical pressure in MPa
- P0 :
-
Saturation pressure in MPa
- R:
-
The universal gas constant in J. K−1 mol−1
- r:
-
The space enclosed by the adsorbate molecule and the adjacent adsorptive site in m
- rm. :
-
The minimum distance between adsorbent-adsorbate molecules in m
- T:
-
Temperature in K
- Tc :
-
Critical temperature in K
- t:
-
The constant
- V:
-
Volume of nitrogen gas in m3
- x :
-
Ratio of the operating pressure and saturation pressure
- Z:
-
Compressibility factor
- ∆ n:
-
Change in number of moles of nitrogen gas in mol.kg−1
- ∆Hads :
-
Change in heat of adsorption in kJ.mol−1
- θ :
-
Fractional surface coverage of nitrogen onto activated carbon
- ϕ :
-
Adsorption potential
- ϕ m :
-
Minimum adsorption potential in J.mol−1
- γ :
-
Correction factor
- ads:
-
Adsorption
- c:
-
Critical
- cal:
-
Calculated
- exp.:
-
Experimental
- f:
-
Final
- i:
-
Initial
References
Anupam K, Palodkar AV, Halder GN (2016) Experimental study on activated carbon – nitrogen pair in a prototype pressure swing adsorption refrigeration system. Heat Mass Transf 52(4):753–761
Anupam K, Chatterjee A, Halder GN, Sarkar S (2011) Experimental investigation of a single-bed pressure swing adsorption refrigeration system towards replacement of halogenated refrigerants. Chem Eng J 171:541–548
Wang DC, Li YH, Li D, Xia YZ, Zhang JP (2010) A review on adsorption refrigeration technology and adsorption deterioration in physical adsorption systems. Renew Sust Energ Rev 14:344–353
Srivastava N, Eames I (1998) A review of adsorbents and adsorbates in solid–vapour adsorption heat pump systems. Appl Therm Eng 18:707–714
Saha BB, El-Sharkawy I, Thorpe R, Critoph RE (2012) Accurate adsorption isotherms of R134a onto activated carbons for cooling and freezing applications. Int J Refrig 35:499–505
Ruthven DM (1984) Principles of adsorption and adsorption processes. A Wiley-Interscience Publication, John Wiley & Sons, New York, pp 49–51
Yalcın N, Sevinc V (2000) Studies of the surface area and porosity of activated carbons prepared from rice husks. Carbon 38:1943–1945
Oh GH, Park CR (2002) Preparation and characteristics of rice-straw-based porous carbons with high adsorption capacity. Fuel 81:327–336
Girgis BS, Yunis SS, Soliman AM (2002) Characteristics of activated carbon from peanut hulls in relation to conditions of preparation. Material Letters 57:164–172
Yang T, Lua AC (2003) Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. J Colloid Interface Sci 267:408–417
Aygun A, Yenisoy-Karakas S, Duman I (2003) Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater 66:189–195
Mohamed KA, Wan Dauda WAN, Chun YY, Donni A (2008) Adsorption capacities of carbon dioxide, oxygen, nitrogen and methane on carbon molecular basket derived from polyethyleneimine impregnation on microporous palm shell activated carbon. Sep Purif Technol 62:609–613
Boonpoke A, Chiarakorn S, Laosiripojana N, Towprayoon S, Chidthaisong A (2011) Synthesis of activated carbon and MCM-41 from bagasse and rice husk and their carbon dioxide adsorption capacity. Journal of Sustainable Energy & Environment 2:77–81
Su W, Zhou L, Zhou YP (2003) Preparation of microporous activated carbon from coconut shells without activating agents. Carbon 41:861–863
Wei SU, Li Z, Yaping Z (2006) Preparation of microporous activated carbon from raw coconut shell by two-step procedure. Chin J Chem Eng 14:266–269
Barrett EP, Joyner LG, Halenda PH (1951) The determination of pore volume and area distributions in porous substances. I Computations from nitrogen isotherms Journal of Americal Chemical Society 73:373–380
Singh K (2001) The use of nitrogen adsorption for the characterization of porous materials. Colloids Surf A Physicochem Eng Asp 187–188:3–9
Ackley MW, Yang RT (1990) Kinetic separation by pressure swing adsorption: method of characteristics model. AICHE J 36:1229–1238
Fatehi AI, Laughlin KF, Hassan MM (1995) Separation of methane-nitrogen mixtures by pressure swing adsorption using carbon molecular sieves. Gas Separation & Purification 9:199–204
Laughlin KF, Hassan MM, Fatehi AI, Zahur M (1993) Rate and equilibrium sorption parameters for nitrogen and methane on carbon molecular sieve. Gas Separation & Purification 7:264–273
Sheikh MA, Hassan MM, Laughlin KF (1996) Adsorption equilibria and rate parameters for nitrogen and methane on Maxsorb activated carbon. Gas Separation & Purification 10(3):161–168
Swain S, Ghosh I (2010) Short communication: conceptual design analysis of a compressor-driven sorption cooling system. International Journal of Research 34:1016–1026
Tzabar N, Grossman G (2008) Nitrogen, methane and ethane sorption on activated carbon. Cryogenics 51:499–508
McEwen J, Hayman JD, Yazaydin AO (2013) A comparative study of CO2, CH4 and N2 adsorption in ZIF-8, zeolite-13X and BPL activated carbon. Chem Phys 412:72–76
Yi H, Li F, Ning P, Tang X, Peng P, Li Y, Deng H (2013) Adsorption separation of CO2, CH4, and N2 on microwave activated carbon. Chem Eng J 215-216:635–642
Shen C, Grande CA, Li P, Yua Y, Rodrigues AE (2010) Adsorption equilibria and kinetics of CO2 and N2 on activated carbon beads. Chem Eng J 160:398–407
Malek A, Farooq S (1996) Comparison of isotherm models for hydrocarbon adsorption on activated carbon. AICHE J 42(11):3191–3201
Chakraborty A, Saha BB, Ng KC, Koyama S, Srinivasan K (2009) Theoretical insight of physical adsorption for a single-component adsorbent + adsorbate system: I thermodynamic property surfaces. Langmuir 25:2204–2211
Yang C, Kaneko K (2002) Adsorption properties of iodine-doped activated carbon fiber. J Colloid Interface Sci 246:34–39
Narayanan KV (2006) A textbook of chemical engineering thermodynamics. Prentice hall of India Pvt. ltd. New Delhi, pp 54-55:194–195
Alexeyer V (1998) Quantitative analysis. Mir Publishers, Moscow
Anupam K, Halder GN, Roy Z, Sarkar SC, Yadav A (2013) A simple calorimeter to measure specific heat of activated carbon prepared for pressure swing adsorption refrigeration system. Can J Chem Eng 91:751–759
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically non uniform surfaces. Chem Rev 60:235–241
Polanyi M (1932) Section III. Theories of the adsorption of gases, a general survey and some additional remarks, introductory paper to section III, Transactions of the Faraday Society 28:316–333
Dubinin M, Astakhov V (1971) Development of the concept of volume filling micro-pore in the adsorption of gases and vapours by microporous adsorbents. Russ Chem Bull 20:13–16
Chakraborty A, Sun B (2014) An adsorption isotherm equation for multi-types adsorption with thermodynamic correctness. Appl Therm Eng 72:190–199
Belton GR (1976) Langmuir adsorption, the Gibbs adsorption isotherm, and interracial kinetics in liquid metal systems. Metall Trans B 7(1):35–42
Masel RI (1996) Principles of adsorption and reaction on solid surfaces. A Wiley-Interscience Publication, John Wiley & Sons, New York, pp 240–241
Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR Journal of Applied Chemistry 3(1):38–45
Chen SG, Yang RT (1994) Theoretical basis for the potential theory adsorption isotherms the Dubinin-Radushkevich and Dubinin-Astakhov equations. Langmuir 10:4244–4249
Dobruskin VK (1998) Micropore volume filling. A condensation approximation approach as a foundation to the Dubinin−Astakhov equation. Langmuir 14:3840–3846
Terzyk AP, Gauden PA, Kowalczyk P (2002) What kind of pore size distribution is assumed in the Dubinin–Astakhov adsorption isotherm equation? Carbon 40:2879–2886
Terzyk AP, Gauden PA, Zawadzki J, Rychlicki G, Wi’sniewski M, Kowalczyk P (2001) Toward the characterization of microporosity of carbonaceous films. J Colloid Interface Sci 243: 183–192.
Nguyen C, Do DD (2001) The Dubinin–Radushkevich equation and the underlying microscopic adsorption description. Carbon 39:1327–1336
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Chakraborty A, Saha BB, Koyama S (2006) On the thermodynamic modeling of the isosteric heat of adsorption and comparison with experiments. Appl Phys Lett 89:171901
Rahman KA, Loh WS, Ng KC (2013) Heat of adsorption and adsorbed phase specific heat capacity of methane/activated carbon system. Procedia Engineering 56:118–125
Acknowledgements
Authors are thankful to the Ministry of Science and Technology, Government of India for the financial assistance through the project DST/ FTYS/ Dec. 9-10. Authors are also grateful to Anoar Ali Khan and Sumit Dhawane, research scholars of Department of Chemical Engineering National Institute of Technology Durgapur for their immense technical support during the execution of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that the research work has no conflict of interest.
Rights and permissions
About this article
Cite this article
Palodkar, A.V., Anupam, K., Roy, Z. et al. High pressure adsorption isotherms of nitrogen onto granular activated carbon for a single bed pressure swing adsorption refrigeration system. Heat Mass Transfer 53, 3155–3166 (2017). https://doi.org/10.1007/s00231-017-2057-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-017-2057-9