Abstract
Objective
Calcitonin gene-related peptide (CGRP) receptor antagonists have been suggested as novel treatments for acute migraine. This study aimed to use meta-analysis to compare the safety and tolerability of five existing oral CGRP receptor antagonists (BI44370TA, MK-3207, rimegepant, telcagepant, and ubrogepant) with that of a placebo or triptans against acute migraine.
Methods
Five prominent databases were searched to identify randomized controlled trials on this topic. The primary safety outcomes of interest were any adverse events (AEs) and treatment-related adverse events (TRAEs), and secondary outcomes were individual events, namely diarrhea, dizziness, dry mouth, fatigue, nausea, paresthesia, somnolence, upper abdominal pain, and vomiting.
Results
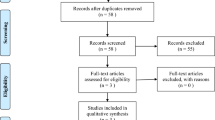
Fifteen studies met the eligibility criteria and were examined in detail. Although, compared to placebo, oral CGRP receptor antagonists significantly increased the incidence of any AEs (risk ratio [RR] = 1.15; 95% confidence interval [CI] = 1.07–1.23), there was no difference in the incidence of TRAEs (RR = 1.18; 95% CI = 1.00–1.38). Moreover, CGRP receptor antagonists were safer than triptans with respect to primary safety outcomes, such as any AEs (RR = 0.78; 95% CI = 0.63–0.98) and TRAEs (RR = 0.68; 95% CI = 0.58–0.79).
Conclusion
Despite oral CGRP receptor antagonists posing a significantly higher risk of AEs when compared to placebo, CGRP receptor antagonists have a favorable safety profile compared to triptans. Our findings inform strategies to enhance safety and tolerability in the treatment of acute migraine.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Kowalska M, Prendecki M, Kozubski W, Lianeri M, Dorszewska J (2016) Molecular factors in migraine. Oncotarget 7(31):50708. https://doi.org/10.18632/oncotarget.9367
Burch R, Rizzoli P, Loder E (2018) The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache: J Head Face Pain 58(4):496–505. https://doi.org/10.1111/head.13281
Viana M, Sances G, Linde M, Ghiotto N, Guaschino E, Allena M, Terrazzino S, Nappi G, Goadsby PJ, Tassorelli C (2017) Clinical features of migraine aura: results from a prospective diary-aided study. Cephalalgia 37(10):979–989. https://doi.org/10.1177/0333102416657147
Charles A (2018) The pathophysiology of migraine: implications for clinical management. Lancet Neurol 17(2):174–182. https://doi.org/10.1016/S1474-4422(17)30435-0
Rasmussen BK, Olesen J (1992) Migraine with aura and migraine without aura: an epidemiological study. Cephalalgia 12(4):221–228. https://doi.org/10.1046/j.1468-2982.1992.1204221.x
de Vries T, Villalón CM, MaassenVanDenBrink A (2020) Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. Pharmacol Ther 211:107528. https://doi.org/10.1016/j.pharmthera.2020.107528
Papademetriou V (2004) Cardiovascular risk assessment and triptans. Headache 44(Suppl 1):S31-39. https://doi.org/10.1111/j.1526-4610.2004.04106.x
Agostoni EC, Barbanti P, Calabresi P, Colombo B, Cortelli P, Frediani F, Geppetti P, Grazzi L, Leone M, Martelletti P, Pini LA, Prudenzano MP, Sarchielli P, Tedeschi G, Russo A (2019) Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain 20(1):92. https://doi.org/10.1186/s10194-019-1038-4
Dos Santos JBR, da Silva MRR (2022) Small molecule CGRP receptor antagonists for the preventive treatment of migraine: a review. Eur J Pharmacol 22:174902. https://doi.org/10.1016/j.ejphar.2022.174902
Yao G, Yu T, Han X, Mao X, Li B (2013) Therapeutic effects and safety of olcegepant and telcagepant for migraine: a meta-analysis. Neural Regen Res 8(10):938–947. https://doi.org/10.3969/j.issn.1673-5374.2013.10.009
Cui XP, Ye JX, Lin H, Mu JS, Lin M (2015) Efficacy, safety, and tolerability of telcagepant in the treatment of acute migraine: a meta-analysis. Pain Pract 15(2):124–131. https://doi.org/10.1111/papr.12158
Hong P, Liu Y (2017) Calcitonin gene-related peptide antagonism for acute treatment of migraine: a meta-analysis. Int J Neurosci 127(1):20–27. https://doi.org/10.3109/00207454.2015.1137915
Canlas KM, Macalintal-Canlas RA, Sakai F (2019) Efficacy of calcitonin gene-related peptide antagonists in the treatment of acute migraine: a systematic review and meta-analysis. Acta Medica Philippina 53(1):44–51. https://doi.org/10.47895/amp.v53i1.223
Gao B, Yang Y, Wang Z, Sun Y, Chen Z, Zhu Y, Wang Z (2019) Efficacy and safety of rimegepant for the acute treatment of migraine: evidence from randomized controlled trials. Front Pharmacol 10:1577. https://doi.org/10.3389/fphar.2019.01577
Chan TLH, Cowan RP, Woldeamanuel YW (2020) Calcitonin gene-related peptide receptor antagonists (gepants) for the acute treatment of nausea in episodic migraine: a systematic review and meta-analysis. Headache 60(7):1489–1499. https://doi.org/10.1111/head.13858
Diao Y, Yang H, Zhou YC, Du B (2020) The efficacy and tolerability of ubrogepant for episodic migraine: a systematic review and meta-analysis. Reserch Square, Preprint:version 2. https://doi.org/10.21203/rs.2.20666/v2
Hong P, Tan T, Liu Y, Xiao J (2020) Gepants for abortive treatment of migraine: a network meta-analysis. Brain Behav 10(8):e01701. https://doi.org/10.1002/brb3.1701
Yang Y, Chen M, Sun Y, Gao B, Chen Z, Wang Z (2020) Safety and efficacy of ubrogepant for the acute treatment of episodic migraine: a meta-analysis of randomized clinical trials. CNS Drugs 34(5):463–471. https://doi.org/10.1007/s40263-020-00715-7
Ha DK, Kim MJ, Han N, Kwak J-H, Baek I-h (2021) Comparative efficacy of oral calcitonin-gene–related peptide antagonists for the treatment of acute migraine: updated meta-analysis. Clin Drug Investigation 41(2):119–132. https://doi.org/10.1007/s40261-020-00997-1
Johnston K, Popoff E, Deighton A, Dabirvaziri P, Harris L, Thiry A, Croop R, Coric V, L’Italien G, Moren J (2021) Comparative efficacy and safety of rimegepant, ubrogepant, and lasmiditan for acute treatment of migraine: a network meta-analysis. Expert Rev Pharmacoecon Outcomes Res 22(1):155–166. https://doi.org/10.1080/14737167.2021.1945444
Kim J, Pak K, Lee GH, Cho JW (2021) Effect of calcitonin gene-related peptide receptor antagonists on migraine treatment: a meta-analysis Reserch Square, Preprint:version 1. https://doi.org/10.1159/000521697
Zhang Z, Shu Y, Diao Y, Du Y, Chen L, Liu Y, Du B (2021) Calcitonin gene-related peptide receptor antagonist ubrogepant for the treatment of acute migraine: a meta-analysis. Medicine (Baltimore) 100(8):e24741. https://doi.org/10.1097/MD.0000000000024741
Ho TW, Ho AP, Chaitman BR, Johnson C, Mathew NT, Kost J, Fan X, Aurora SK, Brandes JL, Fei K, Beebe L, Lines C, Krucoff MW (2012) Randomized, controlled study of telcagepant in patients with migraine and coronary artery disease. Headache 52(2):224–235. https://doi.org/10.1111/j.1526-4610.2011.02052.x
Dodick DW, Lipton RB, Ailani J, Lu K, Finnegan M, Trugman JM, Szegedi A (2019) Ubrogepant for the treatment of migraine. N Engl J Med 381(23):2230–2241. https://doi.org/10.1056/NEJMoa1813049
Lipton RB, Croop R, Stock EG, Stock DA, Morris BA, Frost M, Dubowchik GM, Conway CM, Coric V, Goadsby PJ (2019) Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med 381(2):142–149. https://doi.org/10.1056/NEJMoa1811090
A pharmacokinetic study of MK-1602 in the treatment of acute migraine (MK-1602-007). https://ClinicalTrials.gov/show/NCT01657370. Accessed 30 Jul 2021
Diener HC, Barbanti P, Dahlöf C, Reuter U, Habeck J, Podhorna J (2011) BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia 31(5):573–584. https://doi.org/10.1177/0333102410388435
Hewitt DJ, Aurora SK, Dodick DW, Goadsby PJ, Ge YJ, Bachman R, Taraborelli D, Fan X, Assaid C, Lines C, Ho TW (2011) Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia 31(6):712–722. https://doi.org/10.1177/0333102411398399
Hewitt DJ, Martin V, Lipton RB, Brandes J, Ceesay P, Gottwald R, Schaefer E, Lines C, Ho TW (2011) Randomized controlled study of telcagepant plus ibuprofen or acetaminophen in migraine. Headache 51(4):533–543. https://doi.org/10.1111/j.1526-4610.2011.01860.x
Connor KM, Shapiro RE, Diener HC, Lucas S, Kost J, Fan X, Fei K, Assaid C, Lines C, Ho TW (2009) Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology 73(12):970–977. https://doi.org/10.1212/WNL.0b013e3181b87942
Ho AP, Dahlöf CG, Silberstein SD, Saper JR, Ashina M, Kost JT, Froman S, Leibensperger H, Lines CR, Ho TW (2010) Randomized, controlled trial of telcagepant over four migraine attacks. Cephalalgia 30(12):1443–1457. https://doi.org/10.1177/0333102410370878
Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK (2008) Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 372(9656):2115–2123. https://doi.org/10.1016/S0140-6736(08)61626-8
Connor KM, Aurora SK, Loeys T, Ashina M, Jones C, Giezek H, Massaad R, Williams-Diaz A, Lines C, Ho TW (2011) Long-term tolerability of telcagepant for acute treatment of migraine in a randomized trial. Headache 51(1):73–84. https://doi.org/10.1111/j.1526-4610.2010.01799.x
Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, Lines CR, Rapoport AM (2008) Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology 70(16):1304–1312. https://doi.org/10.1212/01.WNL.0000286940.29755.61
Croop R, Goadsby PJ, Stock DA, Conway CM, Forshaw M, Stock EG, Coric V, Lipton RB (2019) Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet 394(10200):737–745. https://doi.org/10.1016/S0140-6736(19)31606-X
Marcus R, Goadsby PJ, Dodick D, Stock D, Manos G, Fischer TZ (2014) BMS-927711 for the acute treatment of migraine: a double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia 34(2):114–125. https://doi.org/10.1177/0333102413500727
Voss T, Lipton RB, Dodick DW, Dupre N, Ge JY, Bachman R, Assaid C, Aurora SK, Michelson D (2016) A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia 36(9):887–898. https://doi.org/10.1177/0333102416653233
Lipton RB, Dodick DW, Ailani J, Lu K, Finnegan M, Szegedi A, Trugman JM (2019) Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA 322(19):1887–1898. https://doi.org/10.1001/jama.2019.16711
Edvinsson L (2019) Role of CGRP in migraine. Handb Exp Pharmacol 255:121–130. https://doi.org/10.1007/164_2018_201
Goadsby PJ, Holland PR (2019) An update: pathophysiology of migraine. Neurol Clin 37(4):651–671. https://doi.org/10.1016/j.ncl.2019.07.008
Rapoport AM, Lin T (2019) Device profile of the Nerivio™ for acute migraine treatment: overview of its efficacy and safety. Expert Rev Med Devices 16(12):1017–1023. https://doi.org/10.1080/17434440.2019.1695599
Tfelt-Hansen P, Olesen J (2012) Taking the negative view of current migraine treatments: the unmet needs. CNS Drugs 26(5):375–382. https://doi.org/10.2165/11630590-000000000-00000
Yang C-P, Liang C-S, Chang C-M, Yang C-C, Shih P-H, Yau Y-C, Tang K-T, Wang S-J (2021) Comparison of new pharmacologic agents with triptans for treatment of migraine: a systematic review and meta-analysis. JAMA Netw Open 4(10):e2128544. https://doi.org/10.1001/jamanetworkopen.2021.28544
Valentine JC, Pigott TD, Rothstein HR (2010) How many studies do you need? A primer on statistical power for meta-analysis. J Educ Behav Stat 35:215–247. https://doi.org/10.3102/1076998609346961
Jackson D, Turner R (2017) Power analysis for random-effects meta-analysis. Res Synth Methods 8(3):290–302. https://doi-org.ezproxy.library.uq.edu.au/
Funding
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (No. NRF-2022R1F1A1063144). This research was supported by Kyungsung University Research Grants in 2021.
Author information
Authors and Affiliations
Contributions
NH and IB conceived and designed the work. SL, NH, and IB collected the data. SL and IB performed the analysis. NH, CES, NH, and IB gave substantial contributions to data acquisition and interpretation for the work. SL and NH drafted the work. CES and IH revised it critically for important intellectual content. All the authors gave the final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Statement of prior presentation
Results of this study have been partially presented at the 21st annual meeting of the Asian Conference on Clinical Pharmacy, Nagoya, Japan, 11–13 February, 2022.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Oral CGRP receptor antagonists increase the risk of any adverse events compared to placebo, while no difference was found in the incidence of treatment-related adverse events between oral CGRP receptor antagonists and placebo.
• Oral CGRP receptor antagonists are safer than triptans in terms of any adverse events, treatment-related adverse events, dizziness, dry mouth, fatigue, paresthesia, and somnolence.
• Although the safety profile for ubrogepant is more favorable than that of triptans, more stringent safety monitoring is required in patients treated with 100 mg of ubrogepant.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, S., Staatz, C.E., Han, N. et al. Safety evaluation of oral calcitonin-gene–related peptide receptor antagonists in patients with acute migraine: a systematic review and meta-analysis. Eur J Clin Pharmacol 78, 1365–1376 (2022). https://doi.org/10.1007/s00228-022-03347-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03347-6