Abstract
Purpose
Intracellular exposure of tacrolimus (TAC) may be a better marker of therapeutic effect than whole blood exposure. We aimed to evaluate the influence of genetic polymorphism on the pharmacokinetics of TAC in peripheral blood mononuclear cells (PBMCs) and develop limited sampling strategy (LSS) models to estimate the area under the curve (AUC0–12h) in the PBMC of Chinese renal transplant patients.
Methods
Ten blood samples of each of the 23 renal transplant patients were collected 0–12h after 14 (10–18) days of TAC administration. PBMCs were separated and quantified. The TAC level in PBMCs was determined, and pharmacokinetic parameters were estimated by noncompartmental study. The AUC0–12h of TAC in whole blood was estimated by Bayesian approach based on a population pharmacokinetic model established in 65 renal transplant patients. The influence of CYP3A5 and ABCB1 genotypes on exposure was estimated. By applying multiple stepwise linear regression analysis, LSS equations for TAC AUC0–12h in the PMBC of renal transplant patients were established, and the bias and precision of various equations were identified and compared.
Results
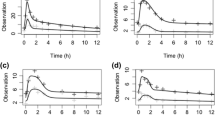
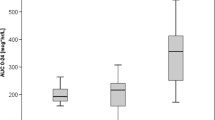
We found a modest correlation between TAC exposure in whole blood and PBMC (r2 = 0.5260). Patients with the CYP3A5 6986GG genotype had a higher AUC0–12h in PBMCs than those with the 6986 AA or GA genotype (P = 0.026). Conversely, patients with the ABCB1 3435TT genotype had a higher AUC0–12h in PBMC than those with the 3435 CC and CT genotypes (P = 0.046). LSS models with 1–4 blood time points were established (r2 = 0.570–0.989). The best model for predicting TAC AUC0–12h was C2–C4–C6–C10 (r2 = 0.989). The model with C0.5–C6 (r2 = 0.849) can be used for outpatients who need monitoring to be performed in a short period.
Conclusions
The CYP3A5 and ABCB1 genotypes impact TAC exposure in PBMCs, which may further alter the effects of TAC. The LSS model consisting of 2–4 time points is an effective approach for estimating full TAC AUC0–12h in Chinese renal transplant patients. This approach may provide convenience and the possibility for clinical monitoring of TAC intracellular exposure.





Similar content being viewed by others
References
Halloran PF, Chang J, Famulski K, Hidalgo LG, Salazar ID, Merino Lopez M et al (2015) Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J Am Soc Nephrol 26(7):1711–1720. https://doi.org/10.1681/ASN.2014060588
Bittersohl H, Schniedewind B, Christians U, Luppa PB (2018) A simple and highly sensitive on-line column extraction liquid chromatography-tandem mass spectrometry method for the determination of protein-unbound tacrolimus in human plasma samples. J Chromatogr A 1547:45–52. https://doi.org/10.1016/j.chroma.2018.03.010
Schreiber SL, Crabtree GR (1992) The mechanism of action of cyclosporin A and FK506. Immunol Today 13(4):136–142. https://doi.org/10.1016/0167-5699(92)90111-j
Spencer CM, Goa KL, Tacrolimus GJC (1997) An update of its pharmacology and clinical efficacy in the management of organ transplantation. Drugs 54(6):925–975. https://doi.org/10.2165/00003495-199754060-00009
Thomson AW, Bonham CA, Zeevi A (1995) Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit 17(6):584–591. https://doi.org/10.1097/00007691-199512000-00007
Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V et al (1995) Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet 29(6):404–430. https://doi.org/10.2165/00003088-199529060-00003
Wallemacq P, Armstrong VW, Brunet M, Haufroid V, Holt DW, Johnston A et al (2009) Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit 31(2):139–152. https://doi.org/10.1097/FTD.0b013e318198d092
Marquet P, Albano L, Woillard JB, Rostaing L, Kamar N, Sakarovitch C et al (2018) Comparative clinical trial of the variability factors of the exposure indices used for the drug monitoring of two tacrolimus formulations in kidney transplant recipients. Pharmacol Res 129:84–94. https://doi.org/10.1016/j.phrs.2017.12.005
Bamoulid J, Staeck O, Halleck F, Dürr M, Paliege A, Lachmann N et al (2015) Advances in pharmacotherapy to treat kidney transplant rejection. Expert Opin Pharmacother 16:1627–1648
Boudjema K, Camus C, Saliba F, Calmus Y, Salamé E, Pageaux G et al (2011) Reduced-dose tacrolimus with mycophenolate mofetil vs. standard-dose tacrolimus in liver transplantation: a randomized study. Am J Transplant 11:965–976
Bahmany S, de Wit LEA, Hesselink DA, van Gelder T, Shuker NM, Baan C et al (2019) Highly sensitive and rapid determination of tacrolimus in peripheral blood mononuclear cells by liquid chromatography-tandem mass spectrometry. Biomed Chromatogr 33(1):e4416. https://doi.org/10.1002/bmc.4416
Capron A, Mourad M, De Meyer M, De Pauw L, Eddour DC, Latinne D et al (2010) CYP3A5 and ABCB1 polymorphisms influence tacrolimus concentrations in peripheral blood mononuclear cells after renal transplantation. Pharmacogenomics 11(5):703–714. https://doi.org/10.2217/pgs.10.43
Capron A, Lerut J, Latinne D, Rahier J, Haufroid V, Wallemacq P (2012) Correlation of tacrolimus levels in peripheral blood mononuclear cells with histological staging of rejection after liver transplantation: preliminary results of a prospective study. Transpl Int 25(1):41–47. https://doi.org/10.1111/j.1432-2277.2011.01365.x
Christians U, Strom T, Zhang YL, Steudel W, Schmitz V, Trump S et al (2006) Active drug transport of immunosuppressants: new insights for pharmacokinetics and pharmacodynamics. Ther Drug Monit 28(1):39–44. https://doi.org/10.1097/01.ftd.0000183385.27394.e7
Tron C, Lemaitre F, Verstuyft C, Petitcollin A, Verdier MC, Bellissant E (2019) Pharmacogenetics of membrane transporters of tacrolimus in solid organ transplantation. Clin Pharmacokinet 58(5):593–613. https://doi.org/10.1007/s40262-018-0717-7
Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W et al (2015) Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther 98(1):19–24. https://doi.org/10.1002/cpt.113
Mendonza AE, Zahir H, Gohh RY, Akhlaghi F (2007) Tacrolimus in diabetic kidney transplant recipients: pharmacokinetics and application of a limited sampling strategy. Ther Drug Monit 29(4):391–398. https://doi.org/10.1097/FTD.0b013e31811f319b
Ting LS, Villeneuve E, Ensom MH (2006) Beyond cyclosporine: a systematic review of limited sampling strategies for other immunosuppressants. Ther Drug Monit 28(3):419–430. https://doi.org/10.1097/01.ftd.0000211810.19935.44
Op den Buijsch RA, van de Plas A, Stolk LM, Christiaans MH, van Hooff JP, Undre NA et al (2007) Evaluation of limited sampling strategies for tacrolimus. Eur J Clin Pharmacol 63(11):1039–1044. https://doi.org/10.1007/s00228-007-0354-9
Wong KM, Shek CC, Chau KF, Li CS (2000) Abbreviated tacrolimus area-under-the-curve monitoring for renal transplant recipients. Am J Kidney Dis 35(4):660–666. https://doi.org/10.1016/s0272-6386(00)70013-8
Chen YH, Zheng KL, Chen LZ, Dai YP, Fei JG, Qiu J et al (2005) Clinical pharmacokinetics of tacrolimus after the first oral administration in combination with mycophenolate mofetil and prednisone in Chinese renal transplant recipients. Transplant Proc 37(10):4246–4250. https://doi.org/10.1016/j.transproceed.2005.11.055
Cheung CY, van der Heijden J, Hoogtanders K, Christiaans M, Liu YL, Chan YH et al (2008) Dried blood spot measurement: application in tacrolimus monitoring using limited sampling strategy and abbreviated AUC estimation. Transpl Int 21(2):140–145. https://doi.org/10.1111/j.1432-2277.2007.00584.x
Chen B, Lu JQ, Shao K, Zhai XH, An HM, Shi HQ et al (2020) Establishment of a liquid chromatography-tandem mass spectrometry method for the determination of immunosuppressant levels in the peripheral blood mononuclear cells of Chinese renal transplant recipients. Ther Drug Monit 42(5):686–694. https://doi.org/10.1097/ftd.0000000000000765
Li ZY, Yan CL, Yan R, Feng ZR (2010) Analytical performance of the Abbott Architect i2000 tacrolimus assay in Chinese patients after renal transplantation. Transplant Proc 42(10):4534–4537. https://doi.org/10.1016/j.transproceed.2010.09.155
Chen B, Fang J, Zhang W, Jin Z, Yu Z, Cai W (2009) Detection of C1236T, G2677T/A, and C3435T polymorphism of MDR1 by amplification refractory mutation system PCR. J Clin Lab Anal 23(2):110–116. https://doi.org/10.1002/jcla.20299
Barraclough KA, Isbel NM, Kirkpatrick CM, Lee KJ, Taylor PJ, Johnson DW et al (2011) Evaluation of limited sampling methods for estimation of tacrolimus exposure in adult kidney transplant recipients. Br J Clin Pharmacol 71(2):207–223. https://doi.org/10.1111/j.1365-2125.2010.03815.x
Staatz CE, Tett SE (2004) Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 43(10):623–653. https://doi.org/10.2165/00003088-200443100-00001
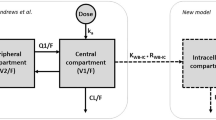
Andrews LM, Li Y, De Winter BCM, Shi YY, Baan CC, Van Gelder T et al (2017) Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert Opin Drug Metab Toxicol 13(12):1225–1236. https://doi.org/10.1080/17425255.2017.1395413
Stienstra NA, Sikma MA, van Dapperen AL, de Lange DW, van Maarseveen EM (2016) Development of a simple and rapid method to measure the free fraction of tacrolimus in plasma using ultrafiltration and LC-MS/MS. Ther Drug Monit 38(6):722–727. https://doi.org/10.1097/ftd.0000000000000351
Han SS, Yang SH, Kim MC, Cho JY, Min SI, Lee JP et al (2016) Monitoring the intracellular tacrolimus concentration in kidney transplant recipients with stable graft function. PLoS ONE 11(4):e0153491. https://doi.org/10.1371/journal.pone.0153491
Lemaitre F, Blanchet B, Latournerie M, Antignac M, Houssel-Debry P, Verdier MC et al (2015) Pharmacokinetics and pharmacodynamics of tacrolimus in liver transplant recipients: inside the white blood cells. Clin Biochem 48(6):406–411. https://doi.org/10.1016/j.clinbiochem.2014.12.018
Kamdem LK, Streit F, Zanger UM, Brockmöller J, Oellerich M, Armstrong VW et al (2005) Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem 51(8):1374–1381. https://doi.org/10.1373/clinchem.2005.050047
Larriba J, Imperiali N, Groppa R, Giordani C, Algranatti S, Redal MA (2010) Pharmacogenetics of immunosuppressant polymorphism of CYP3A5 in renal transplant recipients. Transplant Proc 42(1):257–259. https://doi.org/10.1016/j.transproceed.2009.11.028
Farrell RJ, Menconi MJ, Keates AC, Kelly CP (2002) P-glycoprotein-170 inhibition significantly reduces cortisol and ciclosporin efflux from human intestinal epithelial cells and T lymphocytes. Aliment Pharmacol Ther 16(5):1021–1031. https://doi.org/10.1046/j.1365-2036.2002.01238.x
Picard N, Bergan S, Marquet P, van Gelder T, Wallemacq P, Hesselink DA et al (2016) Pharmacogenetic biomarkers predictive of the pharmacokinetics and pharmacodynamics of immunosuppressive drugs. Ther Drug Monit 38(Suppl 1):S57–S69. https://doi.org/10.1097/ftd.0000000000000255
Staatz CE, Goodman LK, Tett SE (2010) Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II. Clin Pharmacokinet 49(4):207–221. https://doi.org/10.2165/11317550-000000000-00000
Haufroid V (2011) Genetic polymorphisms of ATP-binding cassette transporters ABCB1 and ABCC2 and their impact on drug disposition. Curr Drug Targets 12(5):631–646. https://doi.org/10.2174/138945011795378487
Staatz CE, Goodman LK, Tett SE (2010) Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet 49(3):141–175. https://doi.org/10.2165/11317350-000000000-00000
Vafadari R, Bouamar R, Hesselink DA, Kraaijeveld R, van Schaik RH, Weimar W et al (2013) Genetic polymorphisms in ABCB1 influence the pharmacodynamics of tacrolimus. Ther Drug Monit 35(4):459–465. https://doi.org/10.1097/FTD.0b013e31828c1581
Mahmood I, Miller R (1999) Comparison of the Bayesian approach and a limited sampling model for the estimation of AUC and Cmax: a computer simulation analysis. Int J Clin Pharmacol Ther 37:439–445
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number: 81973387).
Author information
Authors and Affiliations
Contributions
Bing Chen and Pei-jun Zhou designed the study. Xi-han Wang, Xiao-Hui Zhai, and Bing Chen conducted the genotyping and TAC PK study in the PBMCs of renal transplant patients. Kun Shao and Hui-min An analyzed the clinical data of the patients. Xi-han Wang and Bing Chen analyzed the results and drafted the manuscript. All of the authors read and approved the final manuscript. The authors confirm that the PI for this paper is Bing Chen.
Corresponding authors
Ethics declarations
The study was conducted in accordance with the ethical standards of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Ruijin Hospital, which is affiliated with the Shanhai Jiao-Tong University School of Medicine. All the patients who participated in the study provided written informed consent.
Electronic supplementary material
The online version of this article contains supplementary material, which is available to authorized users.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, XH., Shao, K., An, HM. et al. The pharmacokinetics of tacrolimus in peripheral blood mononuclear cells and limited sampling strategy for estimation of exposure in renal transplant recipients. Eur J Clin Pharmacol 78, 1261–1272 (2022). https://doi.org/10.1007/s00228-021-03215-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03215-9