Abstract
Purpose
This single-dose, randomized, open-label, parallel-group, and crossover study assessed pharmacokinetics (PK), pharmacodynamics (PD), and safety of ticagrelor in subjects on hemodialysis versus healthy subjects.
Methods
Hemodialysis subjects were randomized, receiving a single ticagrelor 90-mg dose 1 day post-hemodialysis or just before hemodialysis, with an intervening washout of ≥ 7 days. Healthy subjects (creatinine clearance ≥ 90 mL/min) received a single ticagrelor 90-mg dose. PK, PD (P2Y12 reaction units [PRU], inhibition of platelet aggregation [IPA]), and safety were evaluated.
Results
Twenty-seven subjects (14 hemodialysis, 13 healthy) received ticagrelor. The mean maximum plasma concentration (Cmax) and area under the plasma concentration curve from time zero to infinity (AUC0-∞) of ticagrelor were 598.4 ng/mL and 3256.1 ng·h/mL, respectively, in pre-hemodialysis subjects; 560.3 ng/mL and 3015.1 ng·h/mL, respectively, in post-hemodialysis subjects; and 370.8 ng/mL and 2188.8 ng·h/mL, respectively, in healthy subjects. Cmax and AUC0-∞ of AR-C124910XX, the active metabolite, were 152.3 ng/mL and 1144.2 ng·h/mL, respectively, in pre-hemodialysis subjects; 130.8 ng/mL and 1127.8 ng·h/mL, respectively, in post-hemodialysis subjects; and 111.7 ng/mL and 1000.4 ng·h/mL, respectively, in healthy subjects. Mean IPA time curves over 24 h post-dose were almost indistinguishable for all three treatments. The greatest reduction in mean PRU occurred approximately 2 h post-dose for all three treatments. No safety or tolerability issues were identified.
Conclusion
Hemodialysis resulted in modestly higher exposure to ticagrelor and AR-C124910XX, with no clinically significant effect on PD or tolerability. Accordingly, no dose adjustment is required for hemodialysis patients. Timing of hemodialysis has little impact on ticagrelor PK, or the effect of ticagrelor on IPA.
Similar content being viewed by others
Introduction
Ticagrelor is a direct-acting, reversibly binding oral P2Y12 receptor antagonist that inhibits adenosine diphosphate (ADP)-mediated platelet aggregation [1]. It is given in combination with low-dose aspirin for the secondary prevention of atherothrombotic events in patients with acute coronary syndromes (ACS) [2,3,4].
Ticagrelor is primarily eliminated via hepatic metabolism, with renal excretion playing only a minor role. The primary route of excretion for the active metabolite of ticagrelor is most probably via biliary secretion [5, 6]. The pharmacokinetics (PK), pharmacodynamics (PD), and safety of ticagrelor have been previously studied in a variety of special populations, including subjects with severe renal impairment not on dialysis [7] and subjects with ACS and chronic kidney disease [8]. Compared with subjects with normal renal function, the maximum observed plasma concentration (Cmax) and area under the plasma concentration curve from time zero to infinity (AUC0-∞) of ticagrelor were 20% lower in subjects with severe renal impairment, a difference that is not considered clinically significant. Likewise, PD measures of platelet aggregation appeared to be generally comparable in healthy subjects and those with severe renal impairment, and the safety profile was similar, with no dose adjustments required for renally impaired subjects [7]. When assessed pharmacodynamically versus clopidogrel in patients with ACS and chronic kidney disease, ticagrelor was again shown to be effective and was associated with greater reductions in P2Y12 platelet reaction units (PRU) in the 24 h following loading dose [8].
The PK, PD, and safety of ticagrelor are yet to be definitively established in subjects with end-stage renal disease (ESRD) receiving renal replacement therapy with hemodialysis. Consequently, there are no dosing recommendations for ticagrelor in these patients, despite their increased risk for atherothrombotic and bleeding events when compared with the general population [9,10,11].
This study was conducted to determine the effects of hemodialysis on the PK, PD (based on inhibition of platelet aggregation [IPA] and platelet reactivity, reported as PRU), safety, and tolerability of ticagrelor in subjects with ESRD on hemodialysis, and to provide a rational quantitative basis for ticagrelor dosing recommendations in this patient population.
Methods
Study design and treatment
This was a single-dose, randomized, open-label, parallel-group, and crossover study (NCT02022748) of healthy adult subjects with normal renal function and subjects on hemodialysis enrolled at two study centers (in Lakewood, Colorado and Minneapolis, Minnesota) in the USA. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and in compliance with the International Conference on Harmonisation/Good Clinical Practice guidelines, AstraZeneca bioethics policy, and other applicable regulatory requirements. The study protocol was approved by an institutional review board for each study center, and written informed consent was obtained from all subjects.
Subjects with normal renal function (creatinine clearance ≥ 90 mL/min) were matched by age, weight, and sex to subjects with ESRD on hemodialysis. The main inclusion criteria were men or women aged 18–80 years, body weight ≥ 50 kg, and body mass index (BMI) 18–40 kg/m2, with normal renal function or suffering from ESRD requiring maintenance hemodialysis. Major exclusion criteria included pregnancy; lactation; indication for oral anticoagulant or antiplatelet therapy during the study period (low-dose aspirin was allowed for hemodialysis subjects only); history of ACS within 12 months of study start; contraindication to ticagrelor; increased bleeding risk (platelet count < 100,000/μL) or hemoglobin < 9 g/dL; concomitant therapy with strong cytochrome P450 3A (CYP3A) inhibitors, inducers, or substrates with a narrow therapeutic index within 14 days of study initiation; history of alcohol, substance, or drug abuse within the year preceding the study; and clinically significant laboratory abnormalities as judged by the investigator.
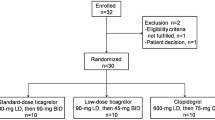
Subjects were screened within 21 days of study initiation (visit 1), which included a physical examination, clinical laboratory testing, 12-lead electrocardiogram (ECG), and relevant medical and surgical history. Renal function was estimated using the Cockcroft-Gault formula [12] and was used to confirm group placement at screening. Laboratory assessments were repeated prior to receiving the study drug. Subjects with normal renal function received a single oral ticagrelor 90-mg dose. Hemodialysis subjects received a single oral ticagrelor 90-mg dose in randomized order either 1 day following hemodialysis (post-hemodialysis) or just prior to the start of hemodialysis (pre-hemodialysis), with crossover to the other regimen after a washout period of at least 7 days.
All subjects were required to fast (2 h for hemodialysis subjects, 8 h overnight for healthy patients) prior to ticagrelor administration and for 2 h post-dose. Ticagrelor was administered with 120 and 240 mL of non-refrigerated water in hemodialysis and healthy subjects, respectively. Subjects sat in an upright or semi-recumbent position for at least 2 h following dosing. Water consumption was restricted from 2 h prior to 2 h following ticagrelor dosing.
Sample collection
Venous blood samples were collected at 1 (for PK only), 2, 4, 6, 12, 24, 36, and 48 h post-dose. PK samples were collected into lithium heparin tubes, chilled, and centrifuged (10 min at 4 °C, relative force of 1500 g) within 30 min of sample collection. PD samples were collected in Greiner Bio-One Vacuette tubes (Greiner Bio-One North America Inc., Monroe, NC, USA), allowed to set for a minimum of 10 min, and assayed within 4 h of collection.
PK and PD sample analyses
Samples for determination of ticagrelor and AR-C124910XX (active metabolite) concentrations in plasma were analyzed by Covance Inc. on behalf of AstraZeneca Research and Development, using an appropriate bioanalytical method [13].
PK analyses
The PK parameters were estimated using standard non-compartmental methods and determined in the subjects who received a dose of ticagrelor, had PK data available, and had no major protocol deviations that might affect the PK of ticagrelor or AR-C124910XX. The following PK parameters were estimated for ticagrelor and AR-C124910XX: Cmax, AUC0-∞, time to reach maximum plasma concentration (tmax), terminal elimination half-life (t1/2), and metabolite/parent drug Cmax and AUC ratios. PK parameters were estimated using WinNonlin version 6.3 (Pharsight Corporation, Mountain View, CA, USA).
PD analyses
The PD profile of ticagrelor was determined in the subjects who received a dose of ticagrelor, had PD data available, and had no major protocol deviations that might affect evaluation of PD, and was based on IPA and platelet reactivity. IPA was measured by light transmission aggregometry using 20-μM ADP as the agonist [14]. The peak IPA (IPAmax) was estimated as the highest IPA (final extent); time to IPAmax was also assessed. The area under the effect curves from 0 to 48 h of final extent IPA (AUEC0–48, IPA) were calculated from IPA time curves using the linear trapezoid rule. IPA percentage was calculated at each time point using the following formula: 100 × (PABL − PAT)/PABL, where PAT is the mean platelet aggregation response at time T, and PABL is the mean response at pre-dose on day 1. The relationship between ticagrelor plasma concentrations and IPA was investigated using a sigmoid maximum effect (Emax) model: IPA = EmaxCγ/(Cγ + EC50γ), where Emax is the maximum effect, EC50 is the concentration that produces 50% of maximal effect, gamma (γ) is the sigmoidicity or shape factor, and C is the plasma concentration of ticagrelor.
Platelet reactivity was measured with the VerifyNow™ P2Y12 assay (Accriva Diagnostics, San Diego, CA, USA), with results reported in PRU. The minimum PRU value (PRUmin) was estimated as the lowest PRU value. The area under the effect curves from 0 to 48 h of final PRU (AUEC0–48, PRU) were calculated from PRU time curves using the linear trapezoid rule.
Safety and tolerability
Assessments of safety and tolerability were based on the safety population (comprising all patients who received a dose of ticagrelor) and included monitoring of adverse events (AEs), physical examination, 12-lead ECGs, vital signs, and laboratory testing. AEs were monitored from time of informed consent until follow-up.
Statistical analyses
PK parameters were summarized using descriptive statistics. All other analyses were generated using SAS® version 9.3 (SAS Institute, Inc., Cary, NC, USA). Treatment comparisons between healthy and hemodialysis subjects were performed using general linear methods with treatment effects. Comparisons of the two hemodialysis regimens were performed using a mixed model. Descriptive analyses were performed for each PD parameter, but the study was not powered for statistical analysis of PD. Sample size determinations were based on accepted standards for this type of investigation.
Results
Demographics and disposition
Twenty-seven subjects (14 hemodialysis subjects, 13 healthy subjects) received the study drug and underwent study procedures between October 2015 and May 2016. Three hemodialysis subjects discontinued treatment (two who received the pre-hemodialysis regimen first and one who received the post-hemodialysis regimen first). In two subjects, discontinuations were due to AEs, while the third subject chose to discontinue the study after receiving the first ticagrelor dose. All 13 healthy subjects completed treatment.
Healthy and hemodialysis subjects were generally well matched. The hemodialysis group comprised 12 men and 2 women, and had a mean (standard deviation [SD]) age of 50.6 (12.5) years and a mean (SD) BMI of 27.8 (4.2) kg/m2. The healthy subject group comprised 10 men and 3 women, with a mean (SD) age and BMI of 43.8 (10.4) years and 28.3 (3.8) kg/m2, respectively. In the hemodialysis group, five subjects were white and nine were black. Of the healthy subjects, six were white, five were black, and two were of other race.
All hemodialysis subjects were on concomitant medication, including treatments for hyperparathyroidism (doxercalciferol) and anemia (saccharated iron oxide, erythropoiesis-stimulating agents), and anticoagulants (heparin). One hemodialysis subject had taken amlodipine within 14 days of study initiation, and although this was recorded as a protocol deviation, it was considered to have negligible impact on the PK of ticagrelor and the subject was included in the PK analysis population. Two healthy subjects reported taking concomitant medications, which included acetaminophen, antacids (calcium carbonate), and aspirin.
Pharmacokinetics
The mean concentration–time profiles of ticagrelor and AR-C124910XX were comparable in hemodialysis and healthy subjects (Fig. 1), and the timing of ticagrelor dosing relative to hemodialysis did not appear to impact the plasma concentration profile.
Ticagrelor was rapidly absorbed, with a median tmax of 2 h in both hemodialysis and healthy subjects, and the mean t1/2 was comparable for all treatments (Table 1). The mean Cmax of ticagrelor was 61% higher in subjects receiving the pre-hemodialysis ticagrelor treatment, compared with that in healthy subjects, and 51% higher following the post-hemodialysis regimen than that in healthy subjects (Table 1, Table S1). Likewise, the mean AUC0-∞ of ticagrelor was 49 and 38% higher when ticagrelor was given pre- and post-hemodialysis, respectively, than that in healthy subjects (Table 1, Table S1). The mean Cmax and AUC0-∞ of ticagrelor were comparable for the two hemodialysis treatments (Table 1). The variability (CV%) for both Cmax and AUC0-∞ of ticagrelor was approximately 50% in hemodialysis subjects, compared with 37% for Cmax and 23% for AUC0-∞ in healthy subjects.
AR-C124910XX was rapidly formed, with a median tmax of 2 h in both hemodialysis and healthy subjects, and the mean t1/2 was similar for all treatments (Table 1). In addition, the mean Cmax of AR-C124910XX was 36% higher following the pre-hemodialysis regimen, compared with that in healthy subjects (Table 1, Table S1). The mean Cmax of AR-C124910XX in patients receiving post-hemodialysis treatment was 17% higher versus that in healthy subjects. The mean AUC0-∞ of AR-C124910XX was 14 and 13% higher in subjects receiving ticagrelor pre- and post-hemodialysis, respectively, than that in healthy subjects (Table 1, Table S1), and like ticagrelor itself, exposure to AR-C124910XX was similar for the two hemodialysis treatment schedules. Cmax of AR-C124910XX values displayed minor variability between the two hemodialysis regimens (pre-hemodialysis, 54%; post-hemodialysis, 38%). The variability of AUC0-∞ of AR-C124910XX was approximately 40% for both hemodialysis treatment schedules. The variability of Cmax and AUC0-∞ of AR-C124910XX was 60 and 33%, respectively, for healthy subjects. The variability of Cmax and AUC0-∞ of AR-C124910XX was considered to be generally similar for hemodialysis versus healthy subjects.
The mean metabolite-to-parent ratios for Cmax (range, 0.26–0.33) and AUC0-∞ (range, 0.38–0.50) were also generally similar between hemodialysis and healthy subjects, suggesting that the presence of ESRD requiring hemodialysis and the timing of hemodialysis have little influence on the metabolic conversion of ticagrelor to AR-C124910XX.
Pharmacodynamics
The mean IPA time curves over 24 h post-dose were almost indistinguishable for all three treatments (Fig. 2). Moreover, final extent IPA was over 90% for all three treatments approximately 2 h after ticagrelor administration (Fig. 2). The mean IPAmax and the AUEC0–48, IPA were generally similar for all three treatments (Table 2), indicating that the presence of ESRD on hemodialysis and the timing of hemodialysis have little influence on the effect of ticagrelor on IPA. The median time to IPAmax was longer when ticagrelor was administered just prior to hemodialysis (4 h) than 1 day after hemodialysis (2 h) or in healthy subjects (2 h) (Table 2). Time to IPAmax displayed much greater variability in hemodialysis subjects (range 2–12 h for both treatments) than in healthy subjects (2 h in all 13 subjects) (Table 2). The Emax model parameters describing the PK/PD relationship between plasma concentrations of ticagrelor and IPA were comparable across all three treatments (Fig. 3), suggesting that the relationship between ticagrelor concentrations and IPA is not altered in ESRD patients on hemodialysis (Table 2).
The mean PRU was decreased following ticagrelor dosing, with the greatest change from baseline occurring approximately 2 h post-dose for all three treatments (Fig. 4). The PRU responses (both absolute values and changes from the baseline) were similar following ticagrelor pre- and post-hemodialysis, while there were much greater responses (lower absolute PRU values) observed in the healthy subjects compared with the hemodialysis subjects (Table 2). The PRU differences at baseline between hemodialysis subjects and healthy subjects contributed to the PRU difference observed at early time points after ticagrelor dosing. Additionally, the difference in effect of ticagrelor on PRU measured as the absolute PRU changes from their baselines is less pronounced which indicates that hemodialysis subjects did not have a greater PRU response when compared with healthy subjects.
Safety
AEs were reported in seven individuals during the study treatment period, distributed across all three treatments (five hemodialysis subjects [three when ticagrelor was administered pre-hemodialysis and two when ticagrelor was administered post-hemodialysis] and two healthy subjects). There were no reports of bleeding or dyspnea in hemodialysis or healthy subjects who received ticagrelor. One subject who received ticagrelor post-hemodialysis reported potentially treatment-related AEs according to the investigator, comprising dizziness, bronchospasm, and nausea. This patient withdrew from the study before receiving the second ticagrelor dose. One other hemodialysis subject withdrew from the study because of a serious AE (thoracic vertebral fracture), which was not considered treatment related. There were no other serious AEs or deaths, and no clinically significant changes in laboratory parameters or vital signs were reported during the study.
Discussion
This study investigated the PK, PD, and safety of ticagrelor in subjects with ESRD on hemodialysis and compared the results with those from healthy subjects with normal renal function. The effects of timing of hemodialysis on the PK of ticagrelor were also investigated as part of the study.
Overall, hemodialysis did not have a clinically significant impact on the exposure to ticagrelor or its active metabolite. The mean Cmax and AUC0-∞ of ticagrelor were 61 and 51%, and 49 and 38% higher in pre- and post-hemodialysis subjects than in healthy subjects, respectively, while the mean Cmax and AUC0-∞ of AR-C124910XX were 36 and 17%, and 14 and 13% higher in pre- and post-hemodialysis subjects, respectively, than that in healthy subjects. Notably, the mean Cmax and AUC0-∞ of ticagrelor and AR-C124910XX were generally similar following a single 90-mg dose of ticagrelor 1 day after hemodialysis and 2 days prior to the next dialysis treatment, indicating that timing of hemodialysis has little effect on exposure to the drug or its metabolite. Moreover, the mean metabolite-to-parent Cmax and AUC0-∞ ratios were comparable following all three treatments, suggesting that hemodialysis has little impact on the metabolic conversion of ticagrelor to AR-C124910XX.
The minimal impact of hemodialysis on ticagrelor PK observed in this study is consistent with the known elimination pathway of ticagrelor, which is predominantly via hepatic metabolism of ticagrelor to AR-C1249XX, followed by biliary secretion [5, 6]. Renal excretion plays only a minor role in the elimination of ticagrelor [5]. Moreover, as ticagrelor is highly protein bound (> 99.8%) [15], dialysis is unlikely to have an appreciable impact on its plasma concentration. The results of this study are also consistent with findings from subjects with severe renal impairment not on hemodialysis, which demonstrated exposure to ticagrelor and AR-C124910XX (20% lower and 17% higher, respectively, vs healthy subjects) to be largely unaffected by renal impairment [7].
IPA responses were also similar between hemodialysis and healthy subjects. Mean IPA time curves for all three treatments were almost indistinguishable, and final extent IPA was > 90% at 2 h post-dose in both hemodialysis and healthy subjects. Likewise, mean IPAmax and AUEC0–48, IPA were similar across the three treatments. Median time to IPAmax was slightly longer when ticagrelor was administered just prior to hemodialysis versus 1 day after hemodialysis or in healthy subjects. However, time to IPAmax values in hemodialysis subjects displayed greater variability than those for healthy subjects, possibly reflecting the small sample size. Nevertheless, the Emax model parameters describing the PK/PD relationship between ticagrelor concentrations and IPA response were comparable across all three treatments. Of particular note, these findings show that hemodialysis subjects did not have greater responses to ticagrelor than healthy subjects, and that the timing of hemodialysis has little influence on the PD effect of ticagrelor on IPA.
For all three treatments, reductions in PRU were observed following ticagrelor dosing, with the greatest reductions observed at 2 h post-dose. The mean AUEC0–48, PRU was of similar magnitude when ticagrelor was administered pre- or post-hemodialysis. However, there appeared to be a greater reduction in PRU in healthy subjects. This finding may be at least partially explained by baseline differences in PRU in hemodialysis versus healthy subjects that may have contributed to the PRU difference observed at early time points after ticagrelor dosing. Importantly, from a safety perspective in this special population, this study highlights that hemodialysis subjects showed no greater PRU response, compared with healthy subjects.
In this single-dose study, safety data were in line with previous studies of ticagrelor in healthy volunteers [16] and subjects with severe renal impairment not on dialysis [7]. AEs were reported in seven subjects across all treatment regimens—three subjects receiving ticagrelor pre-hemodialysis, two subjects receiving ticagrelor post-hemodialysis, and two healthy subjects. Similarly, vital signs and laboratory findings following ticagrelor administration were unremarkable in hemodialysis and healthy subjects, again consistent with previously reported findings in healthy [16] and renally impaired subjects [7].
In conclusion, the slightly higher exposure to ticagrelor and its active metabolite in hemodialysis subjects, compared with healthy subjects, is considered of minimal clinical relevance. Hemodialysis subjects had similar IPA response and no greater PRU response versus healthy subjects, and the safety profile was similar in both cohorts. Based on the PK, PD, and safety findings of this study, no ticagrelor dose adjustment is required for patients with ESRD on hemodialysis. These findings are consistent with the US Food and Drug Administration-approved label for ticagrelor, which suggests that even a twofold increase in exposure to ticagrelor does not warrant a dose adjustment [17]. Furthermore, the timing of hemodialysis has little effect on the PK and PD of ticagrelor.
References
Nawarskas JJ, Snowden SS (2011) Critical appraisal of ticagrelor in the management of acute coronary syndrome. Ther Clin Risk Manag 7:473–488
O’Gara PT, Kushner FG, Ascheim DD et al (2013) 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61:e78–e140
Steg PG, James SK, Atar D et al (2012) ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 33:2569–2619
Roffi M, Patrono C, Collet JP et al (2016) 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 37:267–315
Teng R, Oliver S, Hayes MA, Butler K (2010) Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos 38:1514–1521
Teng R (2015) Ticagrelor: pharmacokinetic, pharmacodynamic and pharmacogenetic profile: an update. Clin Pharmacokinet 54:1125–1138
Butler K, Teng R (2012) Pharmacokinetics, pharmacodynamics, and safety of ticagrelor in volunteers with severe renal impairment. J Clin Pharmacol 52:1388–1398
Wang H, Qi J, Li Y et al (2017) Pharmacodynamics and pharmacokinetics of ticagrelor vs. clopidogrel in patients with acute coronary syndromes and chronic kidney disease. Br J Clin Pharmacol 84:88–96
USRDS United States Renal Data System (2016) USRDS Annual Data Report|Volume 2 ESRD in the United States. Chapter 9: Cardiovascular disease in patients with ESRD. 2.9:465–480
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351:1296–1305
Herzog CA, Asinger RW, Berger AK et al (2011) Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80:572–586
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Sillen H, Cook M, Davis P (2010) Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 878:2299–2306
Butler K, Teng R (2010) Pharmacokinetics, pharmacodynamics, safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol 70:65–77
Butler K, Teng R (2011) Pharmacokinetics, pharmacodynamics, and safety of ticagrelor in volunteers with mild hepatic impairment. J Clin Pharmacol 51:978–987
Teng R, Butler K (2010) Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y(12) receptor antagonist, in healthy subjects. Eur J Clin Pharmacol 66:487–496
AstraZeneca (2016) BRILINTA highlights of prescribing information. BRILINTA (ticagrelor) tablets for oral use:1-10, available at: https://www.azpicentral.com/brilinta/brilinta.pdf
Funding
This study was funded by AstraZeneca.
Author information
Authors and Affiliations
Contributions
R. Teng was involved in developing the study concept and design, interpreting the data, and developing the manuscript. S. Muldowney contributed to the study concept and design, interpretation of data, and development of the manuscript. Y. Zhao was involved in developing the study concept and design, interpreting the data, and developing the manuscript. J.K. Berg was involved in developing the study concept and design, performing study research, interpreting the data, and developing the manuscript. J. Lu contributed to the study concept and design, interpretation of the data, and development of the manuscript. N.D. Khan was involved in developing the study concept and design, interpreting the data, and developing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
S. Muldowney and N.D. Khan are employees and shareholders of AstraZeneca. Y. Zhao is a former consultant to AstraZeneca. R. Teng and J. Lu are former employees of AstraZeneca. J. Berg is an employee of DaVita Clinical Research and a principal investigator in the trial.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Teng, R., Muldowney, S., Zhao, Y. et al. Pharmacokinetics and pharmacodynamics of ticagrelor in subjects on hemodialysis and subjects with normal renal function. Eur J Clin Pharmacol 74, 1141–1148 (2018). https://doi.org/10.1007/s00228-018-2484-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2484-7