Abstract
Purpose
The most common cause of treatment failure in acute lymphoblastic leukaemia (ALL) is the relapse. Genetic polymorphisms of dihydrofolate reductase (DHFR) enzyme affect the response to methotrexate (MTX) treatment. Inter-individual variability exists in the distribution of DHFR variants, and they influence MTX treatment outcome. To the best of our knowledge, there are no genetic studies reported from India, which have explored the influence of DHFR variants on the outcome of MTX treatment. Therefore, we aim to study the influence of DHFR rs408626 (-317A>G) and rs442767 (-680C>A) variants on ALL outcome in South Indian patients.
Methods
A total of 70 ALL patients who were on MTX-based maintenance therapy were recruited for the study. DNA was extracted from leukocytes, and genotyping was done by real-time PCR.
Results
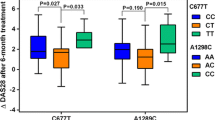
The DHFR-317GG genotype was associated with the increased risk of relapse in patients with ALL (relative risk 2.25, 95 % confidence interval (CI) 1.38 to 3.6, p = 0.02). DHFR-317AA and -680CA genotypes were found to be associated with severe leucopenia (p < 0.05). In Cox regression model, -317GG genotype was found to have lower relapse-free survival (hazard ratio (HR) 2.56, 95 % CI 1.06 to 6.19, p = 0.03) and overall survival (HR 3.72, 95 % CI 1.44 to 9.65, p = 0.007). Similarly, patients with white blood cell (WBC) count >50,000 cells/mm3 were also found to have lower relapse-free survival (HR 2.20, 95 % CI 1.10 to 4.79, p = 0.04) and overall survival (HR 3.30, 95 % CI 1.45 to 7.53, p = 0.004).
Conclusion
The GG genotype of DHFR-317A>G variant is associated with increased risk of ALL relapse and lower overall survival in South Indian population. Both variants of DHFR (-317 AA and -680 CA) are found to be associated with severe leucopenia caused by MTX.

Similar content being viewed by others
References
Pui C-H, Robison LL, Look AT (2008) Acute lymphoblastic leukaemia. Lancet 371:1030–1043. doi:10.1016/S0140-6736(08)60457-2
Hunger SP, Lu X, Devidas M, et al. (2012) Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol 30:1663–1669. doi:10.1200/JCO.2011.37.8018
Cancer Facts & Figures 2011 - acspc-029771.pdf [Internet]. [cited 2015 July 14]. Available from: http://www.cancer.org/acs/groups/content@epidemiologysurveilance/documents/document/acspc-029771.pdf
Magrath I, Shanta V, Advani S, et al. (2005) Treatment of acute lymphoblastic leukaemia in countries with limited resources; lessons from use of a single protocol in India over a twenty year period [corrected]. Eur J Cancer 41:1570–1583. doi:10.1016/j.ejca.2004.11.004
Gaynon PS (2005) Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol 131:579–587. doi:10.1111/j.1365-2141.2005.05773.x
Eden T (2002) Translation of cure for acute lymphoblastic leukaemia to all children. Br J Haematol 118:945–951
Chessells JM, Veys P, Kempski H, et al. (2003) Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol 123:396–405
Locatelli F, Schrappe M, Bernardo ME, Rutella S (2012) How I treat relapsed childhood acute lymphoblastic leukemia. Blood 120:2807–2816. doi:10.1182/blood-2012-02-265884
Rajalekshmy KR, Abitha AR, Anuratha N, Sagar TG (2011) Time trend in frequency of occurrence of major immunophenotypes in paediatric acute lymphoblastic leukemia cases as experienced by Cancer Institute, Chennai, south India during the period 1989-2009. Indian J Cancer 48:310–315. doi:10.4103/0019-509X.84932
Schmiegelow K, Heyman M, Gustafsson G, et al. (2010) The degree of myelosuppression during maintenance therapy of adolescents with B-lineage intermediate risk acute lymphoblastic leukemia predicts risk of relapse. Leukemia 24:715–720. doi:10.1038/leu.2009.303
Schmiegelow K, Heyman M, Kristinsson J, et al. (2009) Oral methotrexate/6-mercaptopurine may be superior to a multidrug LSA2L2 maintenance therapy for higher risk childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study. J Pediatr Hematol Oncol 31:385–392. doi:10.1097/MPH.0b013e3181a6e171
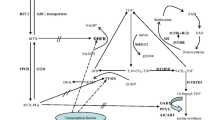
Matherly LH, Taub JW, Ravindranath Y, et al. (1995) Elevated dihydrofolate reductase and impaired methotrexate transport as elements in methotrexate resistance in childhood acute lymphoblastic leukemia. Blood 85:500–509
Serra M, Reverter-Branchat G, Maurici D, et al. (2004) Analysis of dihydrofolate reductase and reduced folate carrier gene status in relation to methotrexate resistance in osteosarcoma cells. Ann Oncol 15:151–160
Göker E, Waltham M, Kheradpour A, et al. (1995) Amplification of the dihydrofolate reductase gene is a mechanism of acquired resistance to methotrexate in patients with acute lymphoblastic leukemia and is correlated with p53 gene mutations. Blood 86:677–684
Galbiatti ALS, Castro R, Caldas HC, et al. (2013) Alterations in the expression pattern of MTHFR, DHFR, TYMS, and SLC19A1 genes after treatment of laryngeal cancer cells with high and low doses of methotrexate. Tumour Biol 34:3765–3771. doi:10.1007/s13277-013-0960-3
Relling MV, Dervieux T (2001) Pharmacogenetics and cancer therapy. Nat Rev Cancer 1:99–108. doi:10.1038/35101056
Cheok MH, Evans WE (2006) Acute lymphoblastic leukaemia: a model for the pharmacogenomics of cancer therapy. Nat Rev Cancer 6:117–129. doi:10.1038/nrc1800
Dulucq S, St-Onge G, Gagné V, et al. (2008) DNA variants in the dihydrofolate reductase gene and outcome in childhood ALL. Blood 111:3692–3700. doi:10.1182/blood-2007-09-110593
Al-Shakfa F, Dulucq S, Brukner I, et al. (2009) DNA variants in region for noncoding interfering transcript of dihydrofolate reductase gene and outcome in childhood acute lymphoblastic leukemia. Clin Cancer Res 15:6931–6938. doi:10.1158/1078-0432.CCR-09-0641
Gómez-Gómez Y, Organista-Nava J, Saavedra-Herrera MV, et al. (2012) Survival and risk of relapse of acute lymphoblastic leukemia in a Mexican population is affected by dihydrofolate reductase gene polymorphisms. Exp Ther Med 3:665–672. doi:10.3892/etm.2012.447
Salazar J, Altés A, del Río E, et al. (2012) Methotrexate consolidation treatment according to pharmacogenetics of MTHFR ameliorates event-free survival in childhood acute lymphoblastic leukaemia. Pharmacogenomics J 12:379–385. doi:10.1038/tpj.2011.25
Tamang R, Singh L, Thangaraj K (2012) Complex genetic origin of Indian populations and its implications. J Biosci 37:911–919
CTCAE_4.03_2010–06-14_QuickReference_5x7.pdf.
Lewis CM (2002) Genetic association studies: design, analysis and interpretation. Brief Bioinformatics 3:146–153
Wang B, Liu M, Yan W, et al. (2013) Association of SNPs in genes involved in folate metabolism with the risk of congenital heart disease. J Matern Fetal Neonatal Med 26:1768–1777. doi:10.3109/14767058.2013.799648
Martinelli M, Girardi A, Cura F, et al. (2014) Evidence of the involvement of the DHFR gene in nonsyndromic cleft lip with or without cleft palate. Eur J Med Genet 57:1–4. doi:10.1016/j.ejmg.2013.12.002
Orjuela MA, Cabrera-Muñoz L, Paul L, et al. (2012) Risk of retinoblastoma is associated with a maternal polymorphism in dihydrofolatereductase (DHFR) and prenatal folic acid intake. Cancer 118:5912–5919. doi:10.1002/cncr.27621
Owen SA, Hider SL, Martin P, et al. (2013) Genetic polymorphisms in key methotrexate pathway genes are associated with response to treatment in rheumatoid arthritis patients. Pharmacogenomics J 13:227–234. doi:10.1038/tpj.2012.7
Sharma S, Das M, Kumar A, et al. (2009) Purine biosynthetic pathway genes and methotrexate response in rheumatoid arthritis patients among north Indians. Pharmacogenet Genomics 19:823–828
Sebro R, Lange C, Laird NM, et al. (2012) Differentiating population stratification from genotyping error using family data. Ann Hum Genet 76:42–52. doi:10.1111/j.1469-1809.2011.00689.x
Cartharius K, Frech K, Grote K, et al. (2005) MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21:2933–2942. doi:10.1093/bioinformatics/bti473
Elmaagacli AH, Koldehoff M, Zakrzewski JL, et al. (2007) Growth factor-independent 1B gene (GFI1B) is overexpressed in erythropoietic and megakaryocytic malignancies and increases their proliferation rate. Br J Haematol 136:212–219. doi:10.1111/j.1365-2141.2006.06407.x
Garçon L, Lacout C, Svinartchouk F, et al. (2005) Gfi-1B plays a critical role in terminal differentiation of normal and transformed erythroid progenitor cells. Blood 105:1448–1455. doi:10.1182/blood-2003-11-4068
Saleque S, Cameron S, Orkin SH (2002) The zinc-finger proto-oncogene Gfi-1b is essential for development of the erythroid and megakaryocytic lineages. Genes Dev 16:301–306. doi:10.1101/gad.959102
Randrianarison-Huetz V, Laurent B, Bardet V, et al. (2010) Gfi-1B controls human erythroid and megakaryocytic differentiation by regulating TGF-beta signaling at the bipotent erythro-megakaryocytic progenitor stage. Blood 115:2784–2795. doi:10.1182/blood-2009-09-241752
Laurent B, Randrianarison-Huetz V, Frisan E, et al. (2012) A short Gfi-1B isoform controls erythroid differentiation by recruiting the LSD1-CoREST complex through the dimethylation of its SNAG domain. J Cell Sci 125:993–1002. doi:10.1242/jcs.095877
Möricke A, Zimmermann M, Reiter A, et al. (2005) Prognostic impact of age in children and adolescents with acute lymphoblastic leukemia: data from the trials ALL-BFM 86, 90, and 95. Klin Padiatr 217:310–320. doi:10.1055/s-2005-872515
Arya LS, Kotikanyadanam SP, Bhargava M, et al. (2010) Pattern of relapse in childhood ALL: challenges and lessons from a uniform treatment protocol. J Pediatr Hematol Oncol 32:370–375. doi:10.1097/MPH.0b013e3181d7ae0d
Wojtuszkiewicz A, Peters GJ, van Woerden NL, et al. (2015) Methotrexate resistance in relation to treatment outcome in childhood acute lymphoblastic leukemia. J Hematol Oncol 8:61. doi:10.1186/s13045-015-0158-9
Radtke S, Zolk O, Renner B, et al. (2013) Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia. Blood 121:5145–5153. doi:10.1182/blood-2013-01-480335
Dervieux T, Greenstein N, Kremer J (2006) Pharmacogenomic and metabolic biomarkers in the folate pathway and their association with methotrexate effects during dosage escalation in rheumatoid arthritis. Arthritis Rheum 54:3095–3103. doi:10.1002/art.22129
Yanada M, Jinnai I, Takeuchi J, et al. (2007) Clinical features and outcome of T-lineage acute lymphoblastic leukemia in adults: a low initial white blood cell count, as well as a high count predict decreased survival rates. Leuk Res 31:907–914. doi:10.1016/j.leukres.2006.08.004
Rujkijyanont P, Kaewinsang S, Monsereenusorn C, Traivaree C (2014) Pediatric acute leukemia: the effect of prognostic factors on clinical outcomes at Phramongkutklao Hospital, Bangkok, Thailand. J Med Assoc Thai 97(Suppl 2):S188–S195
Acknowledgments
We thank the Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India, for providing intramural fund to conduct the study. We also thank Mr. Ravi Prasad for assisting us in the laboratory work.
Conflict of interest
The authors declare that they have no competing interests.
Compliance with Ethical Standards
All procedures performed in the present study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. The study was supported by an intramural grant from JIPMER.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Online source 1
(DOCX 11 kb)
Online source 2
(DOCX 10 kb)
Rights and permissions
About this article
Cite this article
Kodidela, S., Pradhan, S.C., Dubashi, B. et al. Influence of dihydrofolate reductase gene polymorphisms rs408626 (-317A>G) and rs442767 (-680C>A) on the outcome of methotrexate-based maintenance therapy in South Indian patients with acute lymphoblastic leukemia. Eur J Clin Pharmacol 71, 1349–1358 (2015). https://doi.org/10.1007/s00228-015-1930-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1930-z