Abstract
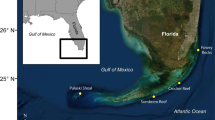
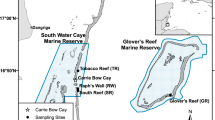
Estimates of contemporary gene flow between or within marine protected areas along the south-east African coast are required to reveal the degree to which coral communities are self-seeding or otherwise connected. Accordingly, we assessed the ecologically relevant (1 or 2 generations) connectivity of two broadcast spawning coral species, Acropora austera and Platygyra daedalea, on reefs in the region, using two types of hyper-variable genetic markers viz. microsatellites and nuclear introns. Analysis of genetic diversity and differentiation provided evidence for the existence of four discrete genetic populations of A. austera and five of P. daedalea in the sampled area. We found higher genetic diversity on northern South African reefs, which suggests these reefs play a role as sinks for putative migrants. Assignment tests that identified migrants to the South African reefs left some individuals unassigned, but assigned the largest proportion of putative recruits to their source reef, providing evidence of high levels of self-seeding and also of an unsampled source reef. Together, the data indicate that South African populations are, at ecological time scales, independent of gene flow from northern coral populations. The findings suggest that the scope of management needed for the protection of the reefs in the region should not be broad in outlook; each reef should be managed as a single unit, part of the whole rather than representative of the whole.



Similar content being viewed by others
References
Almany GR, Connolly SR, Heath DD et al (2009) Connectivity, biodiversity conservation and the design of marine reserve networks for coral reefs. Coral Reefs 28:339–351. doi:10.1007/s00338-009-0484-x
Antao T, Lopes A, Lopes RJ et al (2008) LOSITAN: a workbench to detect molecular adaptation based on a Fst-outlier method. BMC Bioinform 9:323. doi:10.1186/1471-2105-9-323
Baums IB, Paris CB, Cherubin LM (2006) A bio-oceanographic filter to larval dispersal in a reef-building coral. Limnol Oceanogr 51:1969–1981
Beeden R, Maynard JA, Marshall PA et al (2012) A framework for responding to coral disease outbreaks that facilitates adaptive management. Environ Manag 49:1–13. doi:10.1007/s00267-011-9770-9
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc London Ser B Methodol 57:289–300
Benzie J (1999) Major genetic differences between Crown-of-Thorns Starfish (Acanthaster planci) populations in the Indian and Pacific oceans. Evolution (N Y) 53:1782–1795
Bernatchez L, Duchesne P (2000) Individual-based genotype analysis in studies of parentage and population assignment: how many loci, how many alleles? 1. Can J Fish Aquat Sci 57:1–12
Berumen ML, Almany GR, Planes S et al (2012) Persistence of self-recruitment and patterns of larval connectivity in a marine protected area network. Ecol Evol 2:444–452. doi:10.1002/ece3.208
Botsford LW, White JW, Coffroth MA et al (2009) Connectivity and resilience of coral reef metapopulations in marine protected areas: matching empirical efforts to predictive needs. Coral Reefs 28:327–337. doi:10.1007/s00338-009-0466-z
Bryden HL, Beal LM, Duncan LM (2005) Structure and transport of the Agulhas Current and its temporal variability. J Oceanogr 61:479–492
Chapuis M-P, Estoup A (2006) Microsatellite null alleles and estimation of population differentiation. Mol Biol Evol 24:621–631. doi:10.1093/molbev/msl191
Chiazzari B, Macdonald AHH, Schleyer MH (2013) High-latitude connectivity of the scleractinian coral Acropora tenuis in the south-western Indian Ocean, identified using nuclear intron and mitochondrial sequence data. Afr J Mar Sci 35:233–241. doi:10.2989/1814232X.2013.795915
Corander J, Marttinen P, Sirén J, Tang J (2008) Enhanced Bayesian modelling in BAPS software for learning genetic structures of populations. BMC Bioinform 9:539
Cornuet J, Piry S, Luikart G et al (1999) New methods employing multilocus genotypes to select or exclude populations as origins of individuals. Genetics 153:1989–2000
Cowen RK, Paris CB, Srinivasan M (2006) Scaling of connectivity in marine populations. Science 311:522–527
Dmitriev DA, Rakitov RA (2008) Decoding of superimposed traces produced by direct sequencing of heterozygous indels. Plos Comput Biol 4:e1000113. doi:10.1371/journal.pcbi.1000113
Earl DA, VonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361. doi:10.1007/s12686-011-9548-7
Ecoregion WEAM (2004) Towards the establishment of an ecologically representative network of marine protected areas in Kenya, Tanzania and Mozambique. WWF, Dar es Salaam
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol 14:2611–2620. doi:10.1111/j.1365-294X.2005.02553.x
Excoffier L, Smouse PE, Quattro J (1992) Variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data Genetics 131. Genetics 131:479–491
Farhadi A, Farhamand H, Nematollahi MA et al (2013) Mitochondrial DNA population structure of the scalloped lobster Panulirus homarus (Linnaeus 1758) from the West Indian Ocean. ICES J Mar Sci 70:1491–1498. doi:10.1093/icesjms/fst097
Flot J-F (2007) Champuru 1.0: a computer software for unraveling mixtures of two DNA sequences of unequal lengths. Mol Ecol Notes 7:974–977. doi:10.1111/j.1471-8286.2007.01857.x
Frade PR, Reyes-nivia MC, Faria J et al (2010) Semi-permeable species boundaries in the coral genus Madracis: introgression in a brooding coral system. Mol Phylogenet Evol 57:1072–1090. doi:10.1016/j.ympev.2010.09.010
Fratini S, Vannini M (2010) Genetic differentiation in the mud crab Scylla serrata (Decapoda: Portunidae) within the Indian Ocean. J Exp Mar Biol Ecol 272:103–116
Glassom D, Celliers L, Schleyer MH (2006) Coral recruitment patterns at Sodwana Bay, South Africa. Coral Reefs 25:485–492. doi:10.1007/s00338-006-0117-6
Graham EM, Baird AH, Connolly SR (2008) Survival dynamics of scleractinian coral larvae and implications for dispersal. Coral Reefs 27:529–539
Harrison PL, Wallace CC (1990) Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z (ed) Coral Reef. Ecosystems of the worlds. Elsevier, Amsterdam, pp 133–207
Jost L (2008) G ST and its relatives do not measure differentiation. Mol Ecol 17:4015–4026
Kalinowski ST (2004) Counting alleles with rarefaction: private alleles and hierarchical sampling designs. Conserv Genet 5:539–543
Kruger JM, MacFadyen S (2011) Science support within the South African National Parks adaptive management framework. Koedoe 53:1–7. doi:10.4102/koedoe.v53i2.1010
Kruger A, Schleyer MH (1998) Sexual reproduction in the coral Pocillopora verrucosa (Cnidaria: Scleractinia) in KwaZulu-Natal, South Africa. Mar Biol 132:703–710
Lessios HA, Kane J, Robertson DR (2003) Phylogeography of the pantropical sea urchin Tripneustes: contrasting patterns of population structure between oceans. Evolution (N Y) 57:2026–2036
Lutjeharms JRE (2006) Three decades of research on the greater Agulhas Current. Ocean Sci Discuss 3:939–995
Lutjeharms JRE, Biastoch A, van der Werf PM et al (2012) On the discontinuous nature of the Mozambique Current. S Afr J Mar Sci 108:113–117
Macdonald AHH, Schleyer MH, Lamb JM (2008) South east African, high-latitude coral communities, a canary for western Indian Ocean coral reefs? In: Proceedings 11th International Coral Reef Symposium, Ft. Lauderdale, 7–11 July 2008, pp 434–438
Macdonald AHH, Schleyer MH, Lamb JM (2011) Acropora austera connectivity in the south-western Indian Ocean assessed using nuclear intron sequence data. Mar Biol 158:613–621. doi:10.1007/s00227-010-1585-3
Macdonald AHH, Lamb JM, Schleyer MH (2013) Panmixia in East African populations of Platygyra daedalea (Scleractinia: Faviidae). West Indian Ocean J Mar Sci 11:179–191
Mackenzie JB, Munday PL, Willis BL et al (2004) Unexpected patterns of genetic structuring among locations but not colour morphs in Acropora nasuta (Cnidaria; Scleractinia). Mol Ecol 13:9–20
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Márquez LM, Mackenzie JB, Takabayashi M et al (2002) Difficulties in obtaining microsatellites from acroporid corals. Proc 9th Int Coral Reef Symp Bali, Indones 23–27 Oct 2000, pp 139–143
Miller KJ, Howard CJ (2003) Isolation of microsatellites from two species of scleractinian corals. Mol Ecol Notes 1:11–13. doi:10.1046/j.1471-8286.2003.00555.x
Montoya-Maya PH, Macdonald AHH, Schleyer MH (2014a) Estimation of size at first maturity in two South African coral species. Afr J Mar Sci 36:513–516. doi:10.2989/1814232X.2014.980848
Montoya-Maya PH, Macdonald AHH, Schleyer MH (2014b) Cross-amplification and characterization of microsatellite loci in Acropora austera from the south-western Indian Ocean. Genet Mol Res 13:1244–1250. doi:10.4238/2014.February.27.9
Morris T (2009) Physical oceanography of Sodwana Bay and its effect on larval transport and coral bleaching.Msc. Cape Peninsula University of Technology, Cape Town
Muthiga N, Costa A, Motta H, Muhando C, Mwaipopo R, Schleyer MH (2008) Status of coral reefs in East Africa: Kenya, Tanzania, Mozambique and South Africa. In: Willinson C (ed) Status of coral reefs of the world: 2002. Australian Institute of Marine Science, pp 91–104
Muths D, Tessier E, Gouws G et al (2011) Restricted dispersal of the reef fish Myripristis berndti at the scale of the SW Indian Ocean. Mar Ecol Prog Ser 443:167–180. doi:10.3354/meps09394
Muths D, Gouws G, Mwale M et al (2012) Genetic connectivity of the reef fish Lutjanus kasmira at the scale of the western Indian Ocean. Can J Fish Aquat Sci 69:842–853
Muths D, Tessier E, Bourjea J (2015) Genetic structure of the reef grouper Epinephelus merra in the West Indian Ocean appears congruent with biogeographic and oceanographic boundaries. Mar Ecol 36:447–461. doi:10.1111/maec.12153
Nakajima Y, Nishikawa A, Iguchi A, Sakai K (2012) Regional genetic differentiation among northern high-latitude island populations of a broadcast-spawning coral. Coral Reefs 31:1125–1133. doi:10.1007/s00338-012-0932-x
Noreen AME, Harrison PL, van Oppen MJH (2009) Genetic diversity and connectivity in a brooding reef coral at the limit of its distribution. Proc R Soc Biol Sci 276:3927–3935
Nozawa Y, Harrison PL (2002) Larval settlement patterns, dispersal potential, and the effect of temperature on settlement of larvae of the reef coral, Platygyra daedalea, from the Great Barrier Reef. In: Proceedings 9th International Coral Reef Symposium, Bali, 23–27 October 2000
Obura D (2012) The diversity and biogeography of Western Indian Ocean reef-building corals. Plos One 7:e45013. doi:10.1371/journal.pone.0045013
Paetkau D, Calvert W, Stirling I, Strobeck C (1995) Microsatellite analysis of population structure in Canadian polar bears. Mol Ecol 4:347–354. doi:10.1111/j.1365-294X.1995.tb00227.x
Paetkau D, Slade R, Burdens M, Estoup A (2004) Genetic assignment methods for the direct, real-time estimation of migration rate: a simulation-based exploration of accuracy and power. Mol Ecol 13:55–65. doi:10.1046/j.1365-294X.2003.02008.x
Palsbøll PJ, Bérubé M, Allendorf FW (2007) Identification of management units using population genetic data. Trends Ecol Evol 22:11–16. doi:10.1016/j.tree.2006.09.003
Palumbi SR (2003) Population genetics, demographic connectivity, and the design of marine reserves. Ecol Appl 13:146–158
Palumbi SR (2004) Marine reserves and ocean neighborhoods: the spatial scale of marine populations and their management. Annu Rev Environ Resour 29:31–68
Peakall R, Smouse PE (2006) GenAlEx version 6.0: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295. doi:10.1111/j.1471-8286.2005.01155.x
Pearse DE, Crandall KA (2004) Beyond F ST: analysis of population genetic data for conservation. Conserv Genet 5:585–602
Piry S, Alapetite A, Cornuet J et al (2004) GENECLASS2: a software for genetic assignment and first-generation migrant detection. J Hered 95:536–539. doi:10.1093/jhered/esh074
Planes S, Jones GP, Thorrold SR (2009) Larval dispersal connects fish populations in a network of marine protected areas. Proc Natl Acad Sci 106:5693–5697. doi:10.1073/pnas.0808007106
Primmer CR, Koskinen MT, Piironen J (2000) The one that did not get away : individual assignment using microsatellite data detects a case of shing competition fraud. In: Proceedings of the Royal Society BIological Sciences, pp 1699–1704. doi: 10.1098/rspb.2000.1197
Pritchard JK, Stephens P, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rannala B, Mountain JL (1997) Detecting immigration by using multilocus genotypes. Proc Natl Acad Sci USA 94:9197–9210
Reddy MM, Macdonald AHH, Groeneveld JC, Schleyer MH (2014) Phylogeography of the scalloped spiny-lobster Panulirus homarus rubellus in the southwest Indian Ocean. J Crustac Biol 34:773–781. doi:10.1163/1937240X-00002284
Ridgway T, Sampayo EM (2005) Population genetic status of the Western Indian Ocean: what do we know? West Indian Ocean J Mar Sci 4:1–9
Ridgway T, Hoegh-Guldberg O, Ayre DJ (2001) Panmixia in Pocillopora verrucosa from South Africa. Mar Biol 139:175–181
Ridgway T, Riginos C, Davis J, Hoegh-Guldberg O (2008) Genetic connectivity patterns of Pocillopora verrucosa in southern African Marine protected areas. Mar Ecol Prog Ser 354:161–168. doi:10.3354/meps07245
Roberts MJ (2006) Oceanographic environment of the Sodwana Bay coelacanths (Latimeria chalumnae), South Africa. S Afr J Mar Sci 102:435–443
Rocliffe S, Peabody S, Samoilys M, Hawkins JP (2014) Towards a network of locally managed marine areas (LMMAs) in the Western Indian Ocean. Plos One 9:e103000. doi:10.1371/journal.pone.0103000
Rousset F (2008) Genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour 8:103–106
Saenz-Agudelo P, Jones GP, Thorrold SR, Planes S (2009) Estimating connectivity in marine populations: an empirical evaluation of assignment tests and parentage analysis under different gene flow scenarios. Mol Ecol 18:1765–1776. doi:10.1111/j.1365-294X.2009.04109.x
Sale PF, Van Lavieren H, Ablan-Lagman MCA, et al (2010) Preserving reef connectivity: A handbook for marine protected area managers. In: Connectivity Working Group, Coral Reef Targeted Research & Capacity Building for Management Program, UNU-INWEH
Schleyer MH, Celliers L (2005) Modelling reef zonation in the Greater St Lucia Wetland Park, South Africa. Estuar Coast Shelf Sci 63:373–384
Schleyer MH, Kruger A, Celliers L (2008) Long-term community changes on a high-latitude coral reef in the Greater St Lucia Wetland Park, South Africa. Mar Pollut Bull 56:493–502
Selkoe KA, Toonen RJ (2006) Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecol Lett 9:615–629
Silva IC, Mesquita N, Paula J (2010) Lack of population structure in the fiddler crab Uca annulipes along an East African latitudinal gradient: genetic and morphometric evidence. Mar Biol 157:1113–1126. doi:10.1007/s00227-010-1393-9
Sonsthagen SA, Talbot SL, Mccracken KG, Survey USG (2007) Genetic characterization of common eiders breeding in the Yukon-Kuskokwim delta, Alaska. Condor 109:878–893
Souter P, Grahn M (2008) Spatial genetic patterns in lagoonal, reef-slope and island populations of the coral Platygyra daedalea in Kenya and Tanzania Coral Reefs. Coral Reefs 27:433–439
Spalding MD, Fox HE, Allen GR et al (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57:573. doi:10.1641/B570707
Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978–989
Szpiech ZA, Jakobsson M, Rosenberg NA (2008) ADZE: a rarefaction approach for counting alleles private to combinations of populations. Bioinformatics 24:2498–2504. doi:10.1093/bioinformatics/btn478
Tompkins EL, Adger WN (2004) Does adaptive management of natural resources enhance resilience to climate change? Ecol Soc 9:10
Toonen RJ, Hughes S (2001) Increased throughput for fragment analysis on ABI Prism 377 Automated Sequencer using a membrane comb and STRand software. Biotechniques 31:1320–1324
Underwood JN, Smith LD, van Oppen MJH, Gilmour JP (2006) Multiple scales of genetic connectivity in a brooding coral on isolated reefs following catastrophic bleaching. Mol Ecol 16:771–784. doi:10.1111/j.1365-294X.2006.03187.x
Uthicke S, Benzie J (2003) Gene flow and population history in high dispersal marine invertebrates: mitochondrial DNA analysis of Holothuria nobilis (Echinodermata: Holothuroidea) populations from the Indo-Pacific. Mol Ecol 12:2635–2648
Van Der Ven RM, Triest L, De Ryck DJR et al (2016) Population genetic structure of the stony coral Acropora tenuis shows high but variable connectivity in East Africa. J Biogeogr 43:510–519. doi:10.1111/jbi.12643
Visram S, Yang M-C, Pillay RM et al (2010) Genetic connectivity and historical demography of the blue barred parrotfish (Scarus ghobban) in the western Indian Ocean. Mar Biol 157:1475–1487
Vollmer SV, Palumbi SR (2002) Hybridization and the evolution of reef coral diversity. Science 296:2023–2025
Vollmer SV, Palumbi SR (2006) Restricted gene flow in the Caribbean Staghorn Coral Acropora cervicornis: implications for the recovery of endangered reefs. J Hered 98:40–50. doi:10.1093/jhered/esl057
von der Heyden S (2009) Why do we need to integrate population genetics into South African marine protected area planning? Afr J Mar Sci 31:263–269
Wilkinson C, Green A, Almany J, Dionne S (2003) Monitoring coral reef marine protected areas: a practical guide on how monitoring can support effective management of MPAs. Australian Institute of Marine Science and the IUCN Marine Program, Townsville, AU and Gland, Switzerland
Williams ST, Benzie J (1998) Evidence of a biogeographic break between populations of a high dispersal starfish: congruent regions within the Indo-West Pacific defined by color morphs, mtDNA, and allozyme data. Evolution (N Y) 52:87–99
Wood S, Paris CB, Ridgwell A, Hendy EJ (2014) Modelling dispersal and connectivity of broadcast spawning corals at the global scale. Glob Ecol Biogeogr 23:1–11. doi:10.1111/geb.12101
Wright S (1969) Evolution and the genetics of populations. The theory of gene frequencies, vol 2. University of Chicago Press, Chicago
Wright D, Bishop JM, Matthee CA, von der Heyden S (2015) Genetic isolation by distance reveals restricted dispersal across a range of life histories: implications for biodiversity conservation planning across highly variable marine environments. Divers Distrib 21:698–710. doi:10.1111/ddi.12302
Acknowledgments
We thank Justin Hart, Ashley Grimmer, Lola Masse, Mathieu Sere, Stuart Laing and Chris Wikinson for assistance in the field. We also thank Christiaan Labuschagne and Jaco Broere from Inqaba Biotechnical Industries (Pty) Ltd, and Debora Sweby from SASRI for help during the laboratory phase. Thanks are due to Justin Hart and Jessica Escobar-Porras for their help with laboratory work. This work was supported by the Oceanographic Research Institute and the Western Indian Ocean Marine Science Association Grant No. MASMA/CC/2010/06.
Author contributions
PHMM & MHS conceived and designed the research. PHMM conducted the field and laboratory work, analysed the data and wrote and edited the manuscript. MHS assisted in the field, interpretation of the results and writing and editing of the manuscript. AHHM assisted in research design, experimental work, contributed sampling material and assisted in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: T. L. Goulet.
Reviewed by A. Gainsford and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Montoya-Maya, P.H., Schleyer, M.H. & Macdonald, A.H.H. Limited ecologically relevant genetic connectivity in the south-east African coral populations calls for reef-level management. Mar Biol 163, 171 (2016). https://doi.org/10.1007/s00227-016-2939-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2939-2