Abstract
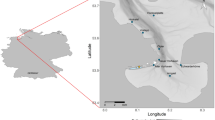
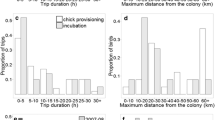
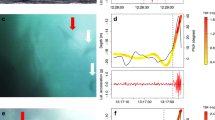
The time seabirds have to forage is restricted while breeding, as time at sea must be balanced against the need to take turns with the partner protecting the nest site or offspring, and timing constraints change once the breeding season is over. Combined geolocator-immersion devices were deployed on eleven Imperial Shags (four males and seven females) in Argentina (43°04′S; 64°2′W) in November 2006 and recovered in November 2007. During the breeding season, females foraged throughout the morning, males exclusively in the afternoon, and variability between individuals was low. Outside the breeding season, both sexes foraged throughout the day, and variability between individuals was high. Timing differences may be explained by higher constraints on foraging or greater demands of parental duties experienced by the smaller sex, females in this case. Sexual differences in reproductive role, feeding habits or proficiency can also lead to segregation in timing of foraging, particularly while breeding.



Similar content being viewed by others
References
Ashmole NP (1963) The regulation of numbers of tropical oceanic birds. Ibis 103:458–473
Bearhop S, Phillips RA, McGill R, Cherel Y, Dawson DA, Croxall JP (2006) Stable isotopes indicate sex-specific and long-term individual foraging specialization in diving seabirds. Mar Ecol Prog Ser 311:157–164
Bernstein NP, Maxson SJ (1981) Notes on molt and seasonably variable characters of the Antarctic Blue-eyed Shag Phalacrocorax atriceps bransfieldensis. Notornis 28:35–39
Bernstein NP, Maxson SJ (1984) Sexually distinct daily activity patterns of Blue-eyed Shags in Antarctica. Condor 86:151–156
Bernstein NP, Maxson SJ (1985) Reproductive energetics of Blue-eyed Shags in Antarctica. Wilson Bull 97:450–462
Catry P, Phillips RA, Croxall JP (2005) Sexual segregation in birds: patterns, processes and implications for conservation. In: Ruckstuhl KE, Neuhaus P (eds) Sexual segregation in vertebrates: ecology of the two sexes. Cambridge University Press, Cambridge, pp 351–378
Chastel O, Weimerskirch H, Jouventin P (1995) Influence of body condition on reproductive decision and reproductive success in the Blue Petrel. Auk 112:964–972
Chaurand T, Weimerskirch H (1994) Incubation routine, body mass regulation and egg neglect in the Blue Petrel Halobaena caerulea. Ibis 136:285–290
Cook TR, Cherel Y, Bost CA, Tremblay Y (2007) Chick-rearing Crozet shags (Phalacrocorax melanogenis) display sex-specific foraging behaviour. Antarct Sci 19:55–63
Daunt F, Afanasyev V, Silk JRD, Wanless S (2005) Extrinsic and intrinsic determinants of winter foraging and breeding phenology in a temperate seabird. Behav Ecol Sociobiol 59:381–388
Dobson FS, Jouventin P (2007) How slow breeding can be selected in seabirds: testing Lack’s hypothesis. Proc R Soc Lond B 274:275–279
Fairbairn DJ, Blanckenhorn WU, Székely T (2007) Sex, size and gender roles evolutionary studies of sexual size dimorphism. Oxford University Press, Oxford
Faraway JJ (2006) Chapter 9: repeated measures and longitudinal data. In: Faraway JJ (ed) Extending the linear model with R: generalized linear, mixed effects and non parametric regression models. Chapman & Hall/CRC, Boca Raton, pp 203–220
Favero M, Casaux R, Silva P, Barrera-Oro E, Coria N (1998) The diet of the Antarctic shag during summer at Nelson Island, Antarctica. Condor 100:112–118
Gaston AJ, Ydenberg RC, Smith GEJ (2007) Ashmole’s halo and population regulation in seabirds. Mar Ornithol 35:119–126
Genzano G, Gilberto D, Bremec C (2011) Benthic survey of natural and artificial reefs off Mar del Plata, Argentina, southwestern Atlantic. Lat Am J Aquat Res 39:553–566
Gómez Laich A, Quintana F, Shepard ELC, Wilson RP (2011) Intersexual differences in the diving behaviour of Imperial Cormorants. J Ornithol 20:1–9
González-Solis J, Croxall JP, Wood AG (2000) Sexual dimorphism and sexual segregation in foraging strategies of northern giant petrels, Macronectes halli, during incubation. Oikos 90:390–398
Grémillet D, Wright G, Lauder A, Carrs DN, Wanless S (2003) Modelling the daily food requirements of wintering great cormorants: a bioenergetics tool for wildlife management. J Appl Ecol 40:266–277
Hedrick AV, Temeles EJ (1989) The evolution of sexual dimorphism in animals: hypotheses and tests. Trends Ecol Evol 4:136–138
Kato A, Watanuki Y, Shaughnessy P, Le Maho Y, Naito Y (1999) Intersexual differences in the diving behaviour of foraging subantarctic cormorant (Phalacrocorax albiventer) and Japanese cormorant (P. filamentosus). Life Sci 322:557–562
Kato A, Watanuki Y, Nishiumi I, Kuroki M, Shaughnessy P, Naito Y (2000) Variation in foraging and parental behavior of king cormorants. Auk 117:718–730
Lack D (1968) Ecological adaptations for breeding in birds. Methuen & Co., London
Lewis S, Benvenuti S, Dall’Antonia L, Griffiths R, Money L, Sherratt TN, Wanless S, Hamer KC (2002) Sex-specific foraging behaviour in a monomorphic seabird. Proc R Soc Lond B 269:1687–1693
Mackley EK, Phillips RA, Silk JRD, Wakefield ED, Afanasyev V, Fox JW, Furness RW (2010) Free as a bird? Activity patterns of albatrosses during the nonbreeding period. Mar Ecol Prog Ser 406:291–303
Malacalza VE, Poretti TI, Bertellotti M (1994) La dieta de Phalacrocorax albiventer en Punta León (Chubut, Argentina) durante la temporada reproductiva. Ornitol Neotrop 5:91–97
Masello JF, Mundry R, Poisbleau M, Demongin L, Voight CC, Wikelski M, Quillfeldt P (2010) Diving seabirds share foraging space and time thin and among species. Ecosph. doi:10.1890/ES10-00103.1
Murray BG (1992) The evolutionary significance of lifetime reproductive success. Auk 109:167–172
Phillips RA, Dawson DA, Ross DJ (2002) Mating patterns and reversed size dimorphism in Southern skuas (Stercorarius skua lonnbergi). Auk 119:858–863
Phillips RA, Silk JRD, Phalan B, Catry P, Croxall JP (2004a) Seasonal sexual segregation in two Thalassarche albatross species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proc R Soc Lond B 271:1283–1291
Phillips RA, Silk JRD, Croxall JP, Afanasyev V, Briggs DR (2004b) Accuracy of geolocation estimates for flying seabirds. Mar Ecol Prog Ser 266:265–272
Phillips RA, McGill RAR, Dawson DA, Bearhop S (2011) Sexual segregation in distribution, diet and trophic level of seabirds: insights from stable isotope analysis. Mar Biol 158:2199–2208
Quillfeldt P, Schroff S, van Noordwijk HJ, Michalik A, Ludynia K, Masello JF (2011) Flexible foraging behaviour of a sexually dimorphic seabird: large males do not always dive deep. Mar Ecol Prog Ser 428:271–287
Quintana F, Wilson R, Dell’Arciprete P, Shepard E, Gómez Laich A (2011) Women from Venus, men from Mars: inter-sex foraging differences in the imperial cormorant Phalacrocorax atriceps a colonial seabird. Oikos 120:350–358
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasmussen PC (1988) Moults of rectrices and body plumage of blue-eyed and king shags (Phalacrocorax atriceps and P. albiventer) and phenology of moults. Notornis 35:129–142
Ropert-Coudert Y, Kato A, Poulin N, Grémillet D (2009) Leg-attached data loggers do not modify the diving performances of a foot-propelled seabird. J Zool 279:294–297
Selander RK (1966) Sexual dimorphism and differential niche utilization in birds. Condor 68:113–151
Serrano-Meneses MA, Székely T (2006) Sexual size dimorphism in seabirds: sexual selection, fecundity selection and differential niche-utilization. Oikos 113:385–394
Shepard ELC, Wilson RP, Quintana F, Gómez Laich A, Forman DW (2009) Pushed for time or saving on fuel: fine-scale energy budgets shed light on currencies in a diving bird. Proc R Soc Lond B 276:3149–3155
Svagelj W, Quintana F (2007) Sexual size dimorphism and sex determination by morphometric measurements in breeding Imperial Shags (Phalacrocorax atriceps). Waterbirds 30:97–102
Svagelj W, Quintana F (2011) Breeding performance of the Imperial Shag (Phalacrocorax atriceps) in relation to year, laying date and nest location. Emu 111:162–165
Tremblay Y, Cook TR, Cherel Y (2005) Time budget and diving behaviour of chick-rearing Crozet shags. Can J Zool 83:971–982
Wallace D, Green SB (2002) Measures designs with linear mixed models. In: Moskowitz DS, Hershberger SL (eds) Modeling intraindividual variability with repeated measures data: methods and applications. Lawrence Erlbaum Associates, publishers, Mahwah, pp 103–170
Wanless S, Harris MP, Morris JA (1995) Factors affecting daily activity budgets of South-Georgian shags during chick rearing at Birds Island, South Georgia. Condor 97:550–558
Wearmouth VJ, Sims DW (2008) Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns mechanisms and conservation implications. In: Sims DW (ed) Advances in marine biology, vol 54, Elsevier Ltd, Amsterdam, pp 107–170
White CR, Butler PJ, Grémillet D, Martin GR (2008) Behavioural strategies of cormorants (Phalacrocoracidae) foraging under challenging light conditions. Ibis 150:231–239
Wilson RP, Quintana F, Hobson VJ (2011) Construction of energy landscapes can clarify the movement and distribution of foraging animals. Proc R Soc Lond B 279:975–980
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R, 1st edn. Springer, Berlin
Acknowledgments
Research was funded by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina, the Wildlife Conservation Society and Agencia de Promoción Científica y Tecnológica to F. Quintana. We wish to thank the British Antarctic Survey for providing GLS devices used in this study. We would also like to thank the Organismo Provincial de Turismo for the permits to work at Punta León, the Centro Nacional Patagónico (CENPAT-CONICET) and Centro Austral de Investigaciones Científicas (CADIC-CONICET) for institutional support, and R. Wilson, M. Uhart, W. Svagelj, J. E. Sala, E. Shepard, and A. Gómez Laich for their assistance in various aspects of this research. S. Harris is supported by a Ph.D. fellowship from CONICET.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Garthe.
Rights and permissions
About this article
Cite this article
Harris, S., Raya Rey, A., Phillips, R.A. et al. Sexual segregation in timing of foraging by imperial shags (Phalacrocorax atriceps): is it always ladies first?. Mar Biol 160, 1249–1258 (2013). https://doi.org/10.1007/s00227-013-2177-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-013-2177-9