Abstract
To construct a nomogram based on clinical factors and paraspinal muscle features to predict vertebral fractures occurring after acute osteoporotic vertebral compression fracture (OVCF). We retrospectively enrolled 307 patients with acute OVCF between January 2013 and August 2022, and performed magnetic resonance imaging of the L3/4 and L4/5 intervertebral discs (IVDs) to estimate the cross-sectional area (CSA) and degree of fatty infiltration (FI) of the paraspinal muscles. We also collected clinical and radiographic data. We used univariable and multivariable Cox proportional hazards models to identify factors that should be included in the predictive nomogram. Post-OVCF vertebral fracture occurred within 3, 12, and 24 months in 33, 69, and 98 out of the 307 patients (10.8%, 22.5%, and 31.9%, respectively). Multivariate analysis revealed that this event was associated with percutaneous vertebroplasty treatment, higher FI at the L3/4 IVD levels of the psoas muscle, and lower relative CSA of functional muscle at the L4/5 IVD levels of the multifidus muscle. Area under the curve values for subsequent vertebral fracture at 3, 12, and 24 months were 0.711, 0.724, and 0.737, respectively, indicating remarkable accuracy of the nomogram. We developed a model for predicting post-OVCF vertebral fracture from diagnostic information about prescribed treatment, FI at the L3/4 IVD levels of the psoas muscle, and relative CSA of functional muscle at the L4/5 IVD levels of the multifidus muscle. This model could facilitate personalized predictions and preventive strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis represents a major public health concern and its importance is growing in the context of the aging global population [1]. One in every two women, and one in every five men, develop a fragility fracture at some point [2]. Osteoporotic vertebral compression fracture (OVCF) is a common and severe consequence of osteoporosis affecting an estimated 1.4 million people per year worldwide [3]. Patients who experience OVCF are at increased risk of subsequent fractures [4,5,6]: approximately 20% of OCVF patients develop new vertebral fractures within 1 year [7,8,9], and approximately 38% within 2 years [10]. This risk of subsequent fractures is greatest within 3 months of a fracture event [11]. The high risk of subsequent fractures during the first 2 years after a fracture is referred to as the imminent risk period [4]. Subsequent vertebral fractures can lead to increased kyphosis, severe deterioration of quality of life, severe functional impairment, and increased mortality [12]. The introduction of fracture liaison services may reduce patient mortality and the risk of subsequent vertebral fracture [13]; it is therefore important to identify risk factors for subsequent vertebral fracture within 3 months and 2 years of the initial fracture event, and to enhance measures and services to improve the prognosis of OVCF.
Many clinical and vertebral factors appear to increase the risk of subsequent vertebral fracture, including advanced age [14, 15], female sex [15], fractures in the thoracolumbar junction [15], high pain scores [16], low bone mineral density (BMD) [17], higher segmental Cobb angle[17], and low vitamin D levels [18]. However, the conclusions of previous studies have been inconsistent, and are only based on clinical and vertebral factors. The paraspinal muscles support and strengthen the spine, thus stabilizing its motion [19]. Impairment of the paraspinal muscles undermines spinal stability. Dysfunction of these muscles is associated with collapse of the fractured vertebra after OVCF [20]. The paraspinal muscles may also play an important role in subsequent vertebral fracture, although this was neglected in most previous studies.
The purpose of this study was to explore potential risk factors for recurrent vertebral fractures and assess the role of paraspinal muscles. Therefore, we developed and validated a nomogram based on clinical factors and paraspinal muscle features for predicting vertebral fractures occurring after acute OVCF.
Materials and Methods
Study Participants
This retrospective study was approved by the institutional review board of Guangzhou Red Cross Hospital (Ethics Approval Number: 2023-329-01, date of approval: 3 January 2023) and the need to obtain informed consent was waived. We reviewed picture archiving and communication system databases to recruit patients diagnosed with acute vertebral compression fracture (VCF) between January 1, 2013 and August 31, 2022. Acute VCF was diagnosed based on the presence of bone marrow oedema and vertebral compression on MRI. We applied the following inclusion criteria: (1) first acute single-segment VCF was at the level of T10–L5, (2) magnetic resonance imaging (MRI) diagnosis of acute VCF, (3) MRI included the L3/4 and L4/5 intervertebral discs (IVDs), (4) follow-up of at least 2 years, (5) availability of lumbar X-ray imaging at the time of initial diagnosis of VCF, and (6) availability of information about BMD based on dual energy X-ray absorptiometry. We adopted the following exclusion criteria: (1) evidence of a previous OVCF; (2) history of vertebral surgery, including vertebroplasty, spinal decompression, or fusion surgery; (3) presence of concurrent spinal tumor or pathological compression fracture; (4) presence of Parkinson’s disease, amyotrophic lateral sclerosis, or other neuromuscular disorders; (5) distraction or rotation injury associated with the vertebral fracture, (6) history of hip fracture and glucocorticoid use, and (7) incomplete follow-up data.
Patients’ data meeting the above criteria were retrospectively analyzed and they were divided into two groups depending on the occurrence (or lack thereof) of a vertebral fracture during follow-up: the subsequent vertebral fracture group (hereafter referred to as the “fracture” group) and non-subsequent vertebral fracture group (hereafter referred to as the “non-fracture” group). The diagnostic standard for establishing occurrence of subsequent vertebral fracture was the presence of a fresh VCF (presence of vertebral bone marrow edema with no occupying effect and no signs of infection) within adjacent and non-adjacent vertebrae on MRI.
Imaging Procedures
MRI and radiographic images of the lumbar spine were obtained using a 1.5 T scanner and digital X-ray system, respectively. Detailed information on these devices and the parameters used during examinations are in Supplementary Material 1.
Data Collection and Image Analysis
Clinical Information
We extracted potential risk factors for subsequent vertebral fracture from the medical records, including age, gender, BMD, fracture time, treatment options for OVCF, and history of anti-osteoporosis treatment. We classified treatment options for OVCF into two categories: percutaneous vertebroplasty (PVP) treatment and non-surgical treatment. PVP is described in detail in Supplementary Material 2. History of anti-osteoporotic treatment was defined as application of anti-osteoporotic medications, including zoledronic acid, denosumab, and teriparatide, and patients who did not apply these medications were those who did not receive anti-osteoporosis treatment. Anti-osteoporotic therapy includes pre-first fracture anti-osteoporotic therapy, post-first fracture anti-osteoporotic therapy, and pre- and post- first fracture anti-osteoporotic therapy.
Radiographic and MRI Image Analysis
Two musculoskeletal radiologists (C.Z. and Y.X.) with 6 and 3 years of experience in musculoskeletal radiology, respectively, independently reviewed all MRIs and radiographs. When there was disagreement, the two radiologists reached a consensus through discussion with a senior radiologist (X.Z.) with 25 years of experience in musculoskeletal radiology.
A lumbar plain radiograph was obtained from patients placed in the lateral standing position during first acute compression fracture. The following imaging parameters were evaluated from radiographs: lumbar lordosis, local kyphotic angle, and fracture location. Lumbar lordosis is defined as the angle between the segment aligned with the L1 upper endplate and the segment aligned with the L5 lower endplate. The local kyphotic angle was measured between vertebrae adjacent to the fracture vertebrae using Cobb’s method [21]. Fracture location was classified into two categories: thoracolumbar junction (T10–L2) and non-thoracolumbar junction (L3–L5).
We evaluated the following parameters from MRI images: cross-sectional area (CSA) of the paraspinal muscle, degree of fatty infiltration (FI) of the paraspinal muscles, compression fracture type, and subcutaneous fat thickness. We estimated CSA and FI using the open-source software ImageJ (version 1.53; National Institutes of Health, Bethesda, MD, USA). We estimated the CSA of the multifidus muscle (MFM), erector spinae muscle (ESM), and psoas muscle (PSM) at the level of the L3/4 and L4/5 IVDs, from the contours of the muscle fascia boundary. These contours were used to delineate regions of interest on T2-weighted images and the total selected muscle area was labeled “TCSA” (for total CSA). We automatically selected regions with different fat signal intensity using the “Moments dark” option of the auto-threshold method. The degree of muscle FI was defined as the ratio of fat area in the muscle to the total area of the muscle. Functional CSA refers to fat-free lean paraspinal muscle [20]. We also calculated the relative CSA (ratio between muscle CSA and CSA of the IVD on the corresponding level) to reduce the effect of body size on muscle parameters [22]. We labeled the relative CSA of total muscle (T) and functional muscle (F) as “rTCSA” and “rFCSA”, respectively. Figure 1 shows the measurement methods and calculation formulas adopted in this study. According to the OF classification [23], compression fractures are divided into five types: OF 1, vertebral body without deformation, only vertebral bone marrow edema apparent; OF 2, deformation, no or slight posterior wall (< 1/5) involvement; OF 3, significant posterior wall deformation (> 1/5); OF 4, loss of integrity of the vertebral framework or pincer fracture; and OF 5, distraction or rotation injury associated with the vertebral fracture. We defined subcutaneous fat thickness as the average thickness of the subcutaneous fat located on the lateral border of the bilateral ESM at the level of the L3/4 IVD.
Image analysis was performed by one of two trained musculoskeletal radiologists with 3 years of subspecialty experience (C.Z. and Y.X.). It takes approximately 2 min per patient to delineate the paraspinal muscles. We randomly selected 50 patients for repeated (2 ×) region of interest delineation, with an interval of 1 week between the two delineations to avoid recall bias.
Statistical Analyses
Statistical analyses were performed using SPSS (version 22.0; IBM Corp., Armonk, NY, USA) and R software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria). Inter-observer and intra-observer measurement reliability was assessed using single-measure intra-class correlation coefficients (ICC) with a two-way random model under absolute agreement. For continuous variables, the Kolmogorov–Smirnov test was used to assess normality, with t tests for those that followed a normal distribution and Mann–Whitney U tests for those that did not. For categorical variables, the Chi-square test and Fisher's exact test were used for comparison. We used univariate Cox analysis to assess the associations between different factors and subsequent vertebral fracture. We then submitted variables with P values < 0.1 in univariate analysis to multivariate analysis (forward stepwise Cox regression). We gradually excluded predictors until all predictors had a P value < 0.05, and defined the associated model as the final predictive model. The final risk factors were incorporated into R software to construct a nomogram prediction model. We used the area under the receiver operating characteristic curve (AUC) over time to assess the discriminatory ability of risk scores at 3 months, 12 months, and 24 months after vertebral fracture. We evaluated model prediction performance using the receiver operating characteristic curve (ROC), and assessed the prediction consistency and clinical utility of the nomogram using calibration curve and decision curve analyses (the predicted risk became closer to the standard curve, the conformity of the model improved). We assessed model accuracy and potential overfit via bootstrap internal validation with 1,000 resampling iterations, and calculated the optimism-corrected C-index.
Results
Patient Characteristics
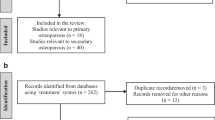
We initially included 1659 patients with acute compression fractures and further based on the inclusion and exclusion criteria, 307 patients were finally included and the detailed process is shown in the flowchart (Fig. 2). A total of 307 patients: 98 in the subsequent vertebral fracture group (median follow-up time: 6.40 months, range 0.23–23.77 months) and 209 in the non-subsequent vertebral fracture group (median follow-up time: 36.60 months, range 24.1–114.93 months). Subsequent vertebral fracture occurred within 3 months in 10.8% (33/307) of patients, 12 months in 22.5% (69/307) of patients, and 24 months in 31.9% (98/307) of patients. Anti-osteoporotic treatment was given to 66 patients, of whom 2 patients received treatment only before the first fracture (1 in the fracture group and 1 the in non-fracture group), 51 patients received treatment only after the first fracture (20 in the fracture group and 31 in the non-fracture group), and 13 patients received anti-osteoporosis treatment both before and after the first fracture (6 in the fracture group and 7 in the non-fracture group). Of the 66 patients, 53 were on zoledronic acid, 12 on teriparatide, and 1 on denosumab. Table 1 provides a summary of the patient characteristics.
Inter- and Intra-observer Agreement and Measurement Reliability
Fourteen area parameters were manually outlined from lumbar spine MR images, that is, left paravertebral muscle area (L-PSM, L-ESM, and L-MFM), right paravertebral muscle area (L-PSM, L-ESM, and L-MFM), and IVD area at the L3/4 IVD level and L4/5 IVD level, respectively, with inter- and intra-observer ICCs greater than 0.90 for all the parameters. Results of the inter- and intra-observer agreement analyses are presented in Supplementary Material 3.
Predictors of Subsequent Vertebral Fracture
The univariable Cox proportional hazard model revealed that the following factors were significantly associated with subsequent vertebral fracture: older age, PVP treatment, lower BMD, and lower rFCSA and higher FI of ESM, MFM, and PSM at the L3/4 and L4/5 IVD levels (Table 2).
Multivariable Cox proportional hazard analysis identified three independent predictors of the risk of subsequent vertebral fracture (Table 2): previous PVP treatment (hazard ratio [HR] 2.95; 95% confidence interval [CI] 1.55–5.60; P = 0.001), higher FIL3/4 of PSM (HR 1.35; 95% CI 1.04–1.75, P = 0.025), and lower rFCSAL4/5 of MFM (HR 1.46; 95% CI 1.46–1.84, P = 0.001).
Development and Assessment of the Predictive Nomogram
We used a stepwise Cox regression model to develop a nomogram for predicting subsequent vertebral fracture within 3, 12, and 24 months after OVCF with a C-index of 0.723 (95% CI 0.646–0.736). Higher total scores for predictors in the nomogram were associated with greater risk of subsequent vertebral fracture (Fig. 3). Internal validation confirmed good performance of the model. AUC values for predicting the risk of subsequent vertebral fracture were 0.711, 0.724, and 0.737 at 3, 12, and 24 months after OVCF, respectively (Fig. 4). After bootstrap validation with 1000 resampling iterations, the bias-corrected C-index values were 0.702, 0.714, and 0.730 at 3, 12, and 24 months after OVCF, respectively, indicating slight systematic overestimation by our model (Fig. 5). Decision curve analysis shows that the nomogram model has sufficient utility for real-world clinical applications (Fig. 6).
Discussion
We developed and internally validated a nomogram based on clinical and paraspinal muscle characteristics to predict vertebral fractures subsequent to an acute osteoporotic vertebral fracture. The nomogram incorporates three factors: PVP treatment, degree of FIL3/4 of PSM, and rFCSAL4/5 of the MFM. The AUC values generated by the nomogram were 0.711, 0.724, and 0.737 at 3, 12, and 24 months after the acute event, respectively. These values demonstrate that prediction accuracy was high, indicating that our nomogram will assist the selection of acute vertebral fracture treatment methods and prediction of vertebral fractures subsequent to OVCF.
OVCF prognosis is impacted by the high rate of subsequent vertebral fracture: approximately 22.5% of the patients in our study experienced subsequent vertebral fracture within 1 year, and 31.9% within 2 years. Mills et al. reported an incidence of subsequent vertebral fracture of 20.7% during 1-year follow-up [24]. Barton et al. demonstrated that the incidence of subsequent vertebral fracture within 2 years can be as high as 38% [10].Their results are broadly consistent with those of our study. The occurrence of subsequent vertebral fracture is affected by many factors. Of these, treatment methods and clinically related factors have been most extensively studied. With regard to treatment methods, vertebroplasty is a surgical treatment method widely used for clinical treatment of OVCF, and its association with subsequent vertebral fractures has been the subject of research. Multivariate Cox proportional hazards analysis confirmed that vertebroplasty was an independent risk factors for subsequent vertebral fracture in our study. Recent guidelines from the American Association of Clinical Endocrinologists [1] do not recommend vertebroplasty because Blasco et al. [25] and Bouza et al. [26] reported that vertebroplasty increases the risk of subsequent vertebral fracture, especially in adjacent vertebrae. A recent study also showed that subsequent vertebral fractures were more frequent, and occurred earlier, in the vertebroplasty group compared with the conservatively treated group [21]. These results are consistent with our own. The authors hypothesize two possible reasons. Firstly, the increased mobility of the patient after vertebroplasty, which increases the risk of subsequent vertebral fracture, and secondly, the possibility that the bone cement may leak into the intervertebral discs, resulting in uneven forces between the vertebrae, making the vertebrae more susceptible to fracture. However, a recent retrospective study of 36,135 patients found no difference in the incidence of subsequent vertebral fracture between patients who received vertebroplasty and those who received non-operative treatment, but anti-osteoporotic medications reduced the rate of subsequent vertebral fracture [24]. In addition, an earlier meta-analysis of 12 controlled clinical trials, randomized controlled trials, and prospective studies by Zhang et al. reached a similar conclusion that no increased risk of vertebral fracture was found after vertebroplasty compared with conservative treatment, especially in vertebrae adjacent to the treated vertebrae [27]. These apparently contradictory results may reflect inconsistent diagnostic evidence for subsequent vertebral fracture. Our diagnosis was based on MRI, whereas other studies only reviewed medical records as evidence of subsequent vertebral fracture. A review of the medical records to make a diagnosis of subsequent vertebral fracture would include some non-fracture cases because osteoporosis also results in loss of vertebral height, which may be misdiagnosed as a fracture.
With regard to clinically related factors, advanced age [14], low BMD [9, 17], and absence of anti-osteoporosis drugs [17, 24] may be associated with subsequent vertebral fracture. In this study, advanced age and low BMD were identified as risk factors by the univariate, but not the multivariate, Cox proportional hazards analysis. This result indicates that other factors play more important roles in the development of subsequent vertebral fractures, and in the process of compensating across risk factors. In this study, only 21.5% of the patients received regular anti-osteoporosis drug treatment. This factor was not different between the fracture and non-fracture groups, which is inconsistent with Mills et al. [24]. We attribute this apparent inconsistency to the small proportion of cases receiving anti-osteoporosis drug treatment in our study.
An important finding of our study was that rFCSA of the MFM at the L4/5 IVD level was an independent risk factor for subsequent vertebral fracture. Among the paraspinal muscles, the MFM is the strongest stabilizer of the lumbar spine [28]. The effect of the bilateral MFM accounts for more than two-thirds of the spine stiffness. This muscle group is able to generate large passive forces, and therefore provides passive resistance to lumbar flexion [29, 30]. Loss of rFCSA within the MFM disrupts the axial stabilization system, which in turn increases axial loading on the spine and relative motion between segments [30, 31]. Hence, we speculate that vertebral attachment to a dysfunctional MFM is less effective against perturbations and thus more susceptible to subsequent vertebral fracture. Consistent with this, previous research reported that incomplete MFM function was associated with bone nonunion after lumbar interbody fusion [22], new compression fracture after vertebroplasty [28], and OVCF [32]. We also found that, although the rFCSA and FI of the MFM at the L3/4 and L4/5 IVD levels were associated with fracture, only rFCSA of the MFM at the L4/5 IVD level was an independent risk factor, even for OVCF at other levels, indicating that this factor best reflects overall MFM function and its influence on subsequent vertebral fracture. This result is also similar to the finding that a low MFM is associated with two or more grades of disc degeneration [33] and increased risk of fall [34], and that pathological changes in the MF typically occur at the L4/5 IVD level [35]. It is consistent with Crawford et al., who reported that the degree of FI of MF at the L4/5 IVD level best represented the degree of FI of the MF in the entire lumbar region of [36]. From the above, it is clear that assessment of MF at the L4/5 IVD level in patients with acute OVCF may be predictive of subsequent vertebral fracture, which would help to identify more vulnerable and higher risk groups in clinical practice.
Another important finding of our study was that FI of the PSM at the L3/4 IVD level was an independent risk factor for subsequent vertebral fracture. The PSM, a core muscle closely related to walking and running, has emerged as a novel, validated surrogate marker for systemic skeletal muscle function [37, 38]. Increased fat infiltration in PSM may reduce functional muscle mass, leading to sarcopenia. Previous studies have also shown that changes in the PSM at the L3/4 IVD level are strongly associated with sarcopenia and significantly associated with many adverse outcomes [38,39,40,41,42]. Sarcopenia is associated with higher risk of falls and fractures [43], and is a major risk factor for loss of independence in older adults [44]. In addition, many studies reported that sarcopenia was associated with vertebral fracture [20, 32, 45,46,47]. The above results are consistent with those of this study.
This study had some limitations. First, it was a retrospective study for which some data could not be obtained or were incomplete. This limitation necessitated the exclusion of some patients, possibly introducing selection bias. Second, the FCSA and FI of the paraspinal muscles were evaluated from T2-weighted MRI data. In the future, we will prospectively adopt magnetic resonance water-fat separation technology for more accurate assessment of the paraspinal muscles. Third, we selectively included cases with new fractures during follow-up confirmed by MRI. Therefore, cases of new fractures without MR examination may have been excluded. Fourth, the use of disc area to adjust for the effect of body size on CSA may not be as authoritative as adjusting for height, but lumbar disc area correlates with height [48], and doing so is reasonable and obtainable on MRI images. Fifth, the study did not include all risk factors that may influence subsequent vertebral fractures, such as falls in the older adults, medications affecting BMD, and frailty.
In conclusion, our results suggest that PVP treatment, low rFCSAL4/5 of the MFM, and excessive FIL3/4 of PSM are independent risk factors for subsequent vertebral fracture. We integrated the above risk factors into a nomogram model, which could aid personalized assessment of recurrent fracture risks after acute vertebral fracture.
Data Availability
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Abbreviations
- OVCF:
-
Osteoporotic vertebral compression fracture
- IVDs:
-
Intervertebral discs
- CSA:
-
Cross-sectional area
- FI:
-
Fatty infiltration
- BMD:
-
Bone mineral density
- MRI:
-
Magnetic resonance imaging
- PVP:
-
Percutaneous vertebroplasty
References
Camacho PM, Petak SM, Binkley N et al (2020) American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract 26:1–46
Lippuner K, Johansson H, Kanis JA, Rizzoli R (2009) Remaining lifetime and absolute 10-year probabilities of osteoporotic fracture in Swiss men and women. Osteoporos Int 20:1131–1140
Johnston CB, Dagar M (2020) Osteoporosis in older adults. Med Clin N Am 104:873–884
Balasubramanian A, Zhang J, Chen L et al (2019) Risk of subsequent fracture after prior fracture among older women. Osteoporos Int 30:79–92
Bliuc D, Alarkawi D, Nguyen TV, Eisman JA, Center JR (2015) Risk of subsequent fractures and mortality in elderly women and men with fragility fractures with and without osteoporotic bone density: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res 30:637–646
Johansson L, Johansson H, Axelsson KF et al (2022) Improved fracture risk prediction by adding VFA-identified vertebral fracture data to BMD by DXA and clinical risk factors used in FRAX. Osteoporos Int 33:1725–1738
Roux C, Cortet B, Bousson V, Thomas T (2021) Vertebroplasty for osteoporotic vertebral fracture. RMD Open 7:e001655
Firanescu CE, de Vries J, Lodder P et al (2018) Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): randomised sham controlled clinical trial. BMJ (Clin Res Ed) 361:k1551
Lindsay R, Silverman SL, Cooper C et al (2001) Risk of new vertebral fracture in the year following a fracture. JAMA 285:320–323
Barton DW, Behrend CJ, Carmouche JJ (2019) Rates of osteoporosis screening and treatment following vertebral fracture. Spine J 19:411–417
Johansson H, Siggeirsdóttir K, Harvey NC et al (2017) Imminent risk of fracture after fracture. Osteoporos Int 28:775–780
Park S-M, Ahn SH, Kim H-Y et al (2020) Incidence and mortality of subsequent vertebral fractures: analysis of claims data of the Korea National Health Insurance Service from 2007 to 2016. Spine J 20:225–233
Vranken L, de Bruin IJA, Driessen AHM et al (2022) Decreased mortality and subsequent fracture risk in patients with a major and hip fracture after the introduction of a fracture liaison service: a 3-year follow-up survey. J Bone Miner Res 37:2025–2032
Banefelt J, Åkesson KE, Spångéus A et al (2019) Risk of imminent fracture following a previous fracture in a Swedish database study. Osteoporos Int 30:601–609
Kanis JA, Johansson H, Harvey NC et al (2021) The effect on subsequent fracture risk of age, sex, and prior fracture site by recency of prior fracture. Osteoporos Int 32:1547–1555
Inose H, Kato T, Ichimura S et al (2021) Risk factors for subsequent vertebral fracture after acute osteoporotic vertebral fractures. Eur Spine J 30:2698–2707
Yamauchi K, Adachi A, Kameyama M et al (2020) A risk factor associated with subsequent new vertebral compression fracture after conservative therapy for patients with vertebral compression fracture: a retrospective observational study. Arch Osteoporos 15:9
Martinez-Ferrer A, Blasco J, Carrasco JL et al (2013) Risk factors for the development of vertebral fractures after percutaneous vertebroplasty. J Bone Miner Res 28:1821–1829
Ignasiak D, Valenzuela W, Reyes M, Ferguson SJ (2018) The effect of muscle ageing and sarcopenia on spinal segmental loads. Eur Spine J 27:2650–2659
Jeon I, Kim SW, Yu D (2022) Paraspinal muscle fatty degeneration as a predictor of progressive vertebral collapse in osteoporotic vertebral compression fractures. Spine J 22:313–320
Park J-S, Park Y-S (2021) Survival analysis and risk factors of new vertebral fracture after vertebroplasty for osteoporotic vertebral compression fracture. Spine J 21:1355–1361
Han G, Zou D, Liu Z et al (2022) Fat infiltration of paraspinal muscles as an independent risk for bone nonunion after posterior lumbar interbody fusion. BMC Musculoskelet Disord 23:232
Schnake KJ, Blattert TR, Hahn P et al (2018) Classification of osteoporotic thoracolumbar spine fractures: recommendations of the spine section of the German Society for Orthopaedics and Trauma (DGOU). Global Spine J 8:46S-49S
Mills ES, Hah RJ, Fresquez Z et al (2022) Secondary fracture rate after vertebral osteoporotic compression fracture is decreased by anti-osteoporotic medication but not increased by cement augmentation. J Bone Joint Surg 104:2178–2185
Blasco J, Martinez-Ferrer A, Macho J et al (2012) Effect of vertebroplasty on pain relief, quality of life, and the incidence of new vertebral fractures: a 12-month randomized follow-up, controlled trial. J Bone Miner Res 27:1159–1166
Bouza C, López-Cuadrado T, Almendro N, Amate JM (2015) Safety of balloon kyphoplasty in the treatment of osteoporotic vertebral compression fractures in Europe: a meta-analysis of randomized controlled trials. Eur Spine J 24:715–723
Zhang H, Xu C, Zhang T, Gao Z, Zhang T (2017) Does Percutaneous vertebroplasty or balloon kyphoplasty for osteoporotic vertebral compression fractures increase the incidence of new vertebral fractures? A meta-analysis. Pain Physician 20:E13–E28
Si F, Yuan S, Zang L et al (2022) Paraspinal muscle degeneration: a potential risk factor for new vertebral compression fractures after percutaneous kyphoplasty. Clin Interv Aging 17:1237–1248
Noonan AM, Brown SHM (2021) Paraspinal muscle pathophysiology associated with low back pain and spine degenerative disorders. JOR Spine 4:e1171
Russo M, Deckers K, Eldabe S et al (2018) Muscle control and non-specific chronic low back pain. Neuromodulation 21:1–9
Ward SR, Kim CW, Eng CM et al (2009) Architectural analysis and intraoperative measurements demonstrate the unique design of the multifidus muscle for lumbar spine stability. J Bone Joint Surg Am 91:176–185
Huang CWC, Tseng IJ, Yang SW, Lin YK, Chan WP (2019) Lumbar muscle volume in postmenopausal women with osteoporotic compression fractures: quantitative measurement using MRI. Eur Radiol 29:4999–5006
Cooley JR, Jensen TS, Kjaer P, Jacques A, Theroux J, Hebert JJ (2022) Spinal degeneration is associated with lumbar multifidus morphology in secondary care patients with low back or leg pain. Sci Rep 12:14676
Ito T, Sakai Y, Yamazaki K, Oikawa M, Morita Y (2019) Relationship between L4/5 lumbar multifidus cross-sectional area ratio and fall risk in older adults with lumbar spinal stenosis: a retrospective study. Geriatrics (Basel, Switzerland) 4:38
Hebert JJ, Kjaer P, Fritz JM, Walker BF (2014) The relationship of lumbar multifidus muscle morphology to previous, current, and future low back pain: a 9-year population-based prospective cohort study. Spine 39:1417–1425
Crawford RJ, Elliott JM, Volken T (2017) Change in fatty infiltration of lumbar multifidus, erector spinae, and psoas muscles in asymptomatic adults of Asian or Caucasian ethnicities. Eur Spine J 26:3059–3067
Hamaguchi Y, Kaido T, Okumura S et al (2016) Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition (Burbank, Los Angeles County, Calif.) 32:1200–1205
Bahat G, Turkmen BO, Aliyev S, Catikkas NM, Bakir B, Karan MA (2021) Cut-off values of skeletal muscle index and psoas muscle index at L3 vertebra level by computerized tomography to assess low muscle mass. Clin Nutr (Edinburgh, Scotland) 40:4360–4365
Modesto AE, Stuart CE, Cho J, Ko J, Singh RG, Petrov MS (2020) Psoas muscle size as a magnetic resonance imaging biomarker of progression of pancreatitis. Eur Radiol 30:2902–2911
Xue Q, Wu J, Ren Y, Hu J, Yang K, Cao J (2021) Sarcopenia predicts adverse outcomes in an elderly population with coronary artery disease: a systematic review and meta-analysis. BMC Geriatr 21:493
Ay M, Cetin H, Cay N (2022) CT evaluation for sarcopenia involving the psoas and paravertebral muscles in patients with total hip arthroplasty. Skeletal Radiol 51:587–593
Tagliafico AS, Bignotti B, Torri L, Rossi F (2022) Sarcopenia: how to measure, when and why. Radiol Med (Torino) 127:228–237
Yeung SSY, Reijnierse EM, Pham VK et al (2019) Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 10:485–500
Dos Santos L, Cyrino ES, Antunes M, Santos DA, Sardinha LB (2017) Sarcopenia and physical independence in older adults: the independent and synergic role of muscle mass and muscle function. J Cachexia Sarcopenia Muscle 8:245–250
Wang WF, Lin CW, Xie CN et al (2019) The association between sarcopenia and osteoporotic vertebral compression refractures. Osteoporos Int 30:2459–2467
Chen Q, Lei C, Zhao T et al (2022) Relationship between sarcopenia/paravertebral muscles and the incidence of vertebral refractures following percutaneous kyphoplasty: a retrospective study. BMC Musculoskelet Disord 23:879
Lidar S, Salame K, Chua M et al (2022) Sarcopenia is an independent risk factor for subsequent osteoporotic vertebral fractures following percutaneous cement augmentation in elderly patients. J Clin Med 11:5778
Oura P, Nurkkala M, Auvinen J, Niinimäki JA-O, Karppinen J, Junno JA (2019) The association of body size, shape and composition with vertebral size in midlife—the Northern Finland birth cohort 1966 study. Sci Rep 9:3944
Acknowledgements
We thank Michael Irvine, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Funding
This study has received funding by the Guangzhou Planned Project of Science and Technology, China [Grant Numbers: 202102010102] (XWZ), Guangzhou Science and Technology Project of Health, China [Grant Numbers: 20231A011021] (YCX), and Guangzhou Science and Technology Project of Health, China [Grant Numbers: 20211A010019] (FX).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Yuchao Xiong, Cici Zhang, Xiaopei Chen, Li Wu, Shaohua Liang, Ye Zhang, Junbing Huang, Wei Guo, Xuwen Zeng, and Fan Xu have no potential conflicts of interest to disclose.
Informed Consent
Informed consent was waived as this study was only retrospective data collection and used de-identified data without any intervention.
Human and Animal Rights
The study was conducted in accordance with the Declaration of Helsinki (revised 2013). The study was approved by our institutional review board (No. 2023-329-01).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiong, Y., Zhang, C., Chen, X. et al. Prediction of Subsequent Vertebral Fracture After Acute Osteoporotic Fractures from Clinical and Paraspinal Muscle Features. Calcif Tissue Int 114, 614–624 (2024). https://doi.org/10.1007/s00223-024-01209-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-024-01209-0