Abstract
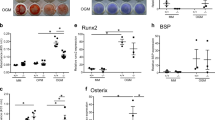
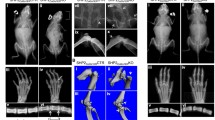
Indian hedgehog (Ihh) is an indispensable paracrine factor for proper tissue patterning, skeletogenesis, and cellular proliferation. Recent genetic studies have revealed critical roles of chondrocyte-derived Ihh in regulating chondrocyte proliferation, hypertrophy and cartilage ossification. However, the functions of Sp7-expressing cell-derived Ihh in osteoblast differentiation and bone formation remain unclear. Sp7 is an essential transcription factor for osteoblast differentiation. In the current study, we generated Sp7-iCre; Ihhfl/fl mice, in which the Ihh gene was specifically deleted in Sp7-expressing cells to investigate the roles of Ihh. Ihh ablation in Sp7-expressing cells resulted in a dwarfism phenotype with severe skeletal dysplasia and lethality at birth, but with normal joint segmentation. Sp7-iCre; Ihhfl/fl mice had fewer osteoblasts, almost no cortical and trabecular bones, smaller skulls, and wider cranial sutures. Additionally, the levels of osteogenesis- and angiogenesis-related genes, and of major bone matrix protein genes were significantly reduced. These results demonstrated that Ihh regulates bone formation in Sp7-expressing cells. Ihh deficiency in primary osteoblasts cultured in vitro inhibited their proliferation, differentiation, and mineralization ability, and reduced the expression of osteogenesis-related genes. Moreover, the deletion of Ihh also attenuated the Bmp2/Smad/Runx2 pathway in E18.5 tibial and primary osteoblasts. The activity of primary osteoblasts in mutant mice was rescued after treatment with rhBMP2. In summary, our data revealed that Ihh in Sp7-expressing cells plays an indispensable role in osteoblast differentiation, mineralization, and embryonic osteogenesis, further implicated that its pro-osteogenic role may be mediated through the canonical Bmp2/Smad/Runx2 pathway.








Similar content being viewed by others
Data Availability
All data used or analyzed during this study are available from the corresponding author on reasonable request.
References
Nakashima K, de Crombrugghe B (2003) Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet 19(8):458–466. https://doi.org/10.1016/S0168-9525(03)00176-8
Berendsen AD, Olsen BR (2015) Bone development. Bone 80:14–18. https://doi.org/10.1016/j.bone.2015.04.035
Mackie EJ, Tatarczuch L, Mirams M (2011) The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol 211(2):109–121. https://doi.org/10.1530/JOE-11-0048
Nilsson O, Marino R, De Luca F, Phillip M, Baron J (2005) Endocrine regulation of the growth plate. Horm Res 64(4):157–165. https://doi.org/10.1159/000088791
van der Eerden BC, Karperien M, Wit JM (2003) Systemic and local regulation of the growth plate. Endocr Rev 24(6):782–801. https://doi.org/10.1210/er.2002-0033
Long F, Ornitz DM (2013) Development of the endochondral skeleton. Cold Spring Harb Perspect Biol 5(1):a008334. https://doi.org/10.1101/cshperspect.a008334
Zuscik MJ, Hilton MJ, Zhang X, Chen D, O’Keefe RJ (2008) Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest 118(2):429–438. https://doi.org/10.1172/JCI34174
de Crombrugghe B, Lefebvre V, Nakashima K (2001) Regulatory mechanisms in the pathways of cartilage and bone formation. Curr Opin Cell Biol 13(6):721–727. https://doi.org/10.1016/s0955-0674(00)00276-3
Bitgood MJ, McMahon AP (1995) Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 172(1):126–138. https://doi.org/10.1006/dbio.1995.0010
Murakami S, Nifuji A, Noda M (1997) Expression of Indian hedgehog in osteoblasts and its posttranscriptional regulation by transforming growth factor-beta. Endocrinology 138(5):1972–1978. https://doi.org/10.1210/endo.138.5.5140
Jemtland R, Divieti P, Lee K, Segre GV (2003) Hedgehog promotes primary osteoblast differentiation and increases PTHrP mRNA expression and iPTHrP secretion. Bone 32(6):611–620. https://doi.org/10.1016/s8756-3282(03)00092-9
Gao B, Guo J, She C, Shu A, Yang M, Tan Z et al (2001) Mutations in IHH, encoding Indian hedgehog, cause brachydactyly type A-1. Nat Genet 28(4):386–388. https://doi.org/10.1038/ng577
Hellemans J, Coucke PJ, Giedion A, De Paepe A, Kramer P, Beemer F et al (2003) Homozygous mutations in IHH cause acrocapitofemoral dysplasia, an autosomal recessive disorder with cone-shaped epiphyses in hands and hips. Am J Hum Genet 72(4):1040–1046. https://doi.org/10.1086/374318
St-Jacques B, Hammerschmidt M, McMahon AP (1999) Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13(16):2072–2086. https://doi.org/10.1101/gad.13.16.2072
Razzaque MS, Soegiarto DW, Chang D, Long F, Lanske B (2005) Conditional deletion of Indian hedgehog from collagen type 2alpha1-expressing cells results in abnormal endochondral bone formation. J Pathol 207(4):453–461. https://doi.org/10.1002/path.1870
Chung UI, Schipani E, McMahon AP, Kronenberg HM (2001) Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest 107(3):295–304. https://doi.org/10.1172/JCI11706
Karp SJ, Schipani E, St-Jacques B, Hunzelman J, Kronenberg H, McMahon AP (2000) Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development 127(3):543–548
Amano K, Densmore MJ, Lanske B (2015) Conditional deletion of Indian hedgehog in limb mesenchyme results in complete loss of growth plate formation but allows mature osteoblast differentiation. J Bone Miner Res 30(12):2262–2272. https://doi.org/10.1002/jbmr.2582
Sun J, Wei X, Li S, Li P, Wei DL, Wei L et al (2018) The effects of Indian hedgehog deletion on mesenchyme cells: inducing intermediate cartilage scaffold ossification to cause growth plate and phalange joint absence, short limb, and dwarfish phenotypes. Stem Cells Dev 27(20):1412–1425. https://doi.org/10.1089/scd.2018.0071
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR et al (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108(1):17–29. https://doi.org/10.1016/s0092-8674(01)00622-5
Rodda SJ, McMahon AP (2006) Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133(16):3231–3244. https://doi.org/10.1242/dev.02480
Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S et al (2010) Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 19(2):329–344. https://doi.org/10.1016/j.devcel.2010.07.010
Pathi S, Rutenberg JB, Johnson RL, Vortkamp A (1999) Interaction of Ihh and BMP/Noggin signaling during cartilage differentiation. Dev Biol 209(2):239–253. https://doi.org/10.1006/dbio.1998.9181
Lenton K, James AW, Manu A, Brugmann SA, Birker D, Nelson ER et al (2011) Indian hedgehog positively regulates calvarial ossification and modulates bone morphogenetic protein signaling. Genesis 49(10):784–796. https://doi.org/10.1002/dvg.20768
Amano K, Okuzaki D, Aikawa T, Kogo M (2020) Indian hedgehog in craniofacial neural crest cells links to skeletal malocclusion by regulating associated cartilage formation and gene expression. FASEB J 34(5):6791–6807. https://doi.org/10.1096/fj.201903269R
Wu M, Chen G, Li YP (2016) TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 26(4):16009. https://doi.org/10.1038/boneres.2016.9
Zhang YW, Yasui N, Ito K, Huang G, Fujii M, Hanai J et al (2000) A RUNX2/PEBP2alpha A/CBFA1 mutation displaying impaired transactivation and Smad interaction in cleidocranial dysplasia. Proc Natl Acad Sci USA 97(19):10549–10554. https://doi.org/10.1073/pnas.180309597
Liu J, Liang C, Guo B, Wu X, Li D, Zhang Z et al (2017) Increased PLEKHO1 within osteoblasts suppresses Smad-dependent BMP signaling to inhibit bone formation during aging. Aging Cell 16(2):360–376. https://doi.org/10.1111/acel.12566
Parr BA, McMahon AP (1995) Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature 374(6520):350–353. https://doi.org/10.1038/374350a0
Droscha CJ, Diegel CR, Ethen NJ, Burgers TA, McDonald MJ, Maupin KA et al (2017) Osteoblast-specific deletion of Hrpt2/Cdc73 results in high bone mass and increased bone turnover. Bone 98:68–78. https://doi.org/10.1016/j.bone.2016.12.006
Byrnes AM, Racacho L, Grimsey A, Hudgins L, Kwan AC, Sangalli M et al (2009) Brachydactyly A-1 mutations restricted to the central region of the N-terminal active fragment of Indian Hedgehog. Eur J Hum Genet 17(9):1112–1120. https://doi.org/10.1038/ejhg.2009.18
Inada M, Yasui T, Nomura S, Miyake S, Deguchi K, Himeno M et al (1999) Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev Dyn 214(4):279–290. https://doi.org/10.1002/(SICI)1097-0177(199904)214:4%3c279::AID-AJA1%3e3.0.CO;2-W
Karaplis AC (2002) Embryonic development of bone and the molecular regulation of intramembranous and endochondral bone formation— science direct [J]. Principles Bone Biol (Second Edition) 1:33–58. https://doi.org/10.1016/B978-012098652-1.50105-0
Yang L, Tsang KY, Tang HC, Chan D, Cheah KS (2014) Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A 111(33):12097–12102. https://doi.org/10.1073/pnas.1302703111
Maeda Y, Nakamura E, Nguyen MT, Suva LJ, Swain FL, Razzaque MS et al (2007) Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc Natl Acad Sci USA 104(15):6382–6387. https://doi.org/10.1073/pnas.0608449104
Ono N, Ono W, Nagasawa T, Kronenberg HM (2014) A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol 16(12):1157–1167. https://doi.org/10.1038/ncb3067
Capurro MI, Xu P, Shi W, Li F, Jia A, Filmus J (2008) Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev Cell 14(5):700–711. https://doi.org/10.1016/j.devcel.2008.03.006
Li N, Wei L, Liu X, Bai H, Ye Y, Li D et al (2019) A frizzled-like cysteine-rich domain in glypican-3 mediates Wnt binding and regulates hepatocellular carcinoma tumor growth in mice. Hepatology 70(4):1231–1245. https://doi.org/10.1002/hep.30646
Feng M, Gao W, Wang R, Chen W, Man YG, Figg WD et al (2013) Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc Natl Acad Sci USA 110(12):E1083–E1091. https://doi.org/10.1073/pnas.1217868110
Dwivedi PP, Grose RH, Filmus J, Hii CS, Xian CJ, Anderson PJ et al (2013) Regulation of bone morphogenetic protein signalling and cranial osteogenesis by Gpc1 and Gpc3. Bone 55(2):367–376. https://doi.org/10.1016/j.bone.2013.04.013
Acknowledgements
Ihhfl/fl mice were gifted by Beate Lanske (Harvard School of Dental Medicine). We thank Linrui Zhang for her help in statistical analysis. The authors have declared that no competing interest exists. This work was supported by the National Natural Science Foundation of China (NSFC81772415, NSFC81772867, NSFC82172503).
Funding
This work was supported by the National Natural Science Foundation of China (NSFC81772415, NSFC81772867, NSFC82172503).
Author information
Authors and Affiliations
Contributions
Designed the research: YW, PL and XW; Performed research: YW; Analyzed data: YW, ZD, JS; Provided technical assistance: CW, LG, HL; Wrote the manuscript: YW.
Corresponding author
Ethics declarations
Conflict of Interest
YunFei Wang, Zhengquan Dong, Ruijia Yang, Sujing Zong, Xiaochun Wei, Chunfang Wang, Li Guo, Jian Sun, Haoqian Li, and Pengcui Li have declared that no competing interest exists.
Human and Animal Rights and Informed Consent
All experiments were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Shanxi Medical University (Approval Number:2017003). There are no human sujects in the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Dong, Z., Yang, R. et al. Inactivation of Ihh in Sp7-Expressing Cells Inhibits Osteoblast Proliferation, Differentiation, and Bone Formation, Resulting in a Dwarfism Phenotype with Severe Skeletal Dysplasia in Mice. Calcif Tissue Int 111, 519–534 (2022). https://doi.org/10.1007/s00223-022-00999-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-00999-5