Abstract
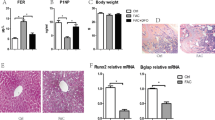
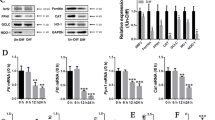
Iron overload is closely associated with osteoporosis, the potential cellular mechanism involved in decreased osteoblast differentiation and increased osteoclast formation. However, the effect of iron overload on the biological behavior in osteocytes has not been reported. This study aims to investigate the changes of osteocytic activity, apoptosis, and its regulation on osteoclastogenesis in response to iron overload. MLO-Y4 osteocyte-like cells and primary osteocytes from mice were processed with ferric ammonium citrate (FAC) and deferoxamine (DFO), the conditioned medium (CM) was harvested and co-cultured with Raw264.7 cells and bone marrow-derived macrophages (BMDMs) to induce them to differentiate into osteoclasts. Osteocyte apoptosis, osteoclast differentiation, osteocytic gene expression and protein secretion of receptor activator of nuclear factor κB ligand (RANKL) and osteoprotegerin (OPG) was examined. Excessive iron has a toxic effect on MLO-Y4 osteocyte-like cells. Increased cell apoptosis in MLO-Y4 cells and primary osteocytes was induced by iron overload. The osteoclastic formation, differentiation-related gene expression, and osteoclastic bone‐resorption capability were significantly increased after treated with the CM from iron overload‐exposed osteocytes. Excessive iron exposure significantly promoted the gene expression and protein secretion of the RANKL in MLO‐Y4 cells. Addition of RANKL-blocking antibody completely abolished the increase of osteoclast formation and bone resorption capacity induced by the CM from osteocytes exposed to excessive iron. Moreover, the pan-caspase apoptosis inhibitor, QVD (quinolyl-valyl-O-methylaspartyl-[-2,6-difluorophenoxy]-methylketone) was used to inhibit osteocyte apoptosis. The results showed osteocyte apoptosis induced by iron overload was reduced by QVD and accompanied by the decrease of soluble RANKL (sRANKL) in supernatant. The increase of osteoclast formation and bone resorption capacity induced by the CM from osteocytes exposed to excessive iron was significantly decreased by QVD. These results indicated that iron overload-induced osteocyte apoptosis is required to regulate osteoclast differentiation by increasing osteocytic RANKL production. This study, for the first time, reveals the indirect effect of iron overload on osteoclast differentiation through regulating osteocytes.







Similar content being viewed by others
References
Jeney V (2017) Clinical Impact and cellular mechanisms of iron overload-associated bone loss. Front Pharmacol 8:77. https://doi.org/10.3389/fphar.2017.00077
Tsay J, Yang Z, Ross FP, Cunningham-Rundles S, Lin H, Coleman R, Mayer-Kuckuk P et al (2010) Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood 116:2582–2589. https://doi.org/10.1182/blood-2009-12-260083
Yang Q, Jian J, Abramson SB, Huang X (2011) Inhibitory effects of iron on bone morphogenetic protein 2-induced osteoblastogenesis. J Bone Miner Res 26:1188–1196. https://doi.org/10.1002/jbmr.337
Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, Aburatani H et al (2009) Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15:259–266. https://doi.org/10.1038/nm.1910
Wang L, Fang B, Fujiwara T, Krager K, Gorantla A, Li C, Feng JQ et al (2018) Deletion of ferroportin in murine myeloid cells increases iron accumulation and stimulates osteoclastogenesis in vitro and in vivo. J Biol Chem 293:9248–9264. https://doi.org/10.1074/jbc.RA117.000834
Kang H, Yan Y, Jia P, Yang K, Guo C, Chen H, Qi J et al (2016) Desferrioxamine reduces ultrahigh-molecular-weight polyethylene-induced osteolysis by restraining inflammatory osteoclastogenesis via heme oxygenase-1. Cell Death Dis 7:e2435. https://doi.org/10.1038/cddis.2016.339
Baschant U, Rauner M, Bulycheva E, Weidner H, Roetto A, Platzbecker U, Hofbauer LC (2016) Wnt5a is a key target for the pro-osteogenic effects of iron chelation on osteoblast progenitors. Haematologica 101:1499–1507. https://doi.org/10.3324/haematol.2016.144808
Robling AG, Bonewald LF (2020) The osteocyte: new insights. Annu Rev Physiol 82:485–506. https://doi.org/10.1146/annurev-physiol-021119-034332
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA (2011) Matrix-embedded cells control osteoclast formation. Nat Med 17:1235–1241. https://doi.org/10.1038/nm.2448
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF et al (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17:1231–1234. https://doi.org/10.1038/nm.2452
Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T (2006) Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res 21:605–615. https://doi.org/10.1359/jbmr.060107
Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB (2012) Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone 50:1115–1122. https://doi.org/10.1016/j.bone.2012.01.025
Kennedy OD, Laudier DM, Majeska RJ, Hui B, Sun HB, Schaffler MB (2014) Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone 64:132–137. https://doi.org/10.1016/j.bone.2014.03.049
Cabahug-Zuckerman P, Frikha-Benayed D, Majeska RJ, Tuthill A, Yakar S, Judex S, Schaffler MB (2016) Osteocyte apoptosis caused by hindlimb unloading is required to trigger osteocyte RANKL production and subsequent resorption of cortical and trabecular bone in mice femurs. J Bone Miner Res 31:1356–1365. https://doi.org/10.1002/jbmr.2807
Yuan Y, Xu F, Cao Y, Xu L, Yu C, Yang F, Zhang P et al (2018) Iron accumulation leads to bone loss by inducing mesenchymal stem cell apoptosis through the activation of Caspase3. Biol Trace Elem Res. https://doi.org/10.1007/s12011-018-1388-9
Tian Q, Wu S, Dai Z, Yang J, Zheng J, Zheng Q, Liu Y (2016) Iron overload induced death of osteoblasts in vitro: involvement of the mitochondrial apoptotic pathway. PeerJ 4:e2611. https://doi.org/10.7717/peerj.2611
Stern AR, Stern MM, Van Dyke ME, Jahn K, Prideaux M, Bonewald LF (2012) Isolation and culture of primary osteocytes from the long bones of skeletally mature and aged mice. Biotechniques 52:361–373. https://doi.org/10.2144/0000113876
Wang P, Tang C, Wu J, Yang Y, Yan Z, Liu X, Shao X et al (2019) Pulsed electromagnetic fields regulate osteocyte apoptosis, RANKL/OPG expression, and its control of osteoclastogenesis depending on the presence of primary cilia. J Cell Physiol 234:10588–10601. https://doi.org/10.1002/jcp.27734
van Swelm RPL, Wetzels JFM, Swinkels DW (2019) The multifaceted role of iron in renal health and disease. Nat Rev Nephrol. https://doi.org/10.1038/s41581-019-0197-5
Nurtjahja-Tjendraputra E, Fu D, Phang JM, Richardson DR (2006) Iron chelation regulates cyclin D1 expression via the proteasome: a link to iron deficiency-mediated growth suppression. Blood 109:4045–4054. https://doi.org/10.1182/blood-2006-10-047753
Zhao GY, Zhao LP, He YF, Li GF, Gao C, Li K, Xu YJ (2012) A comparison of the biological activities of human osteoblast hFOB1.19 between iron excess and iron deficiency. Biol Trace Elem Res 150:487–495. https://doi.org/10.1007/s12011-012-9511-9
Yang F, Yang L, Li Y, Yan G, Feng C, Liu T, Gong R et al (2017) Melatonin protects bone marrow mesenchymal stem cells against iron overload-induced osteogenic differentiation dysfunction and senescence. J Pineal Res. https://doi.org/10.1111/jpi.12422
Lertsuwan K, Nammultriputtar K, Nanthawuttiphan S, Phoaubon S, Lertsuwan J, Thongbunchoo J, Wongdee K et al (2018) Ferrous and ferric differentially deteriorate proliferation and differentiation of osteoblast-like UMR-106 cells. Biometals 31:873–889. https://doi.org/10.1007/s10534-018-0130-6
Cen WJ, Feng Y, Li SS, Huang LW, Zhang T, Zhang W, Kong WD et al (2018) Iron overload induces G1 phase arrest and autophagy in murine preosteoblast cells. J Cell Physiol 233:6779–6789. https://doi.org/10.1002/jcp.26405
Yao X, Jing X, Guo J, Sun K, Deng Y, Zhang Y, Guo F et al (2019) Icariin protects bone marrow mesenchymal stem cells against iron overload induced dysfunction through mitochondrial fusion and fission, PI3K/AKT/mTOR and MAPK Pathways. Front Pharmacol 10:163. https://doi.org/10.3389/fphar.2019.00163
Yang M, Chan S, Ye J, ChiFung CG (2013) TPO exerts a protective effect on iron-overload induces apoptosis in cardiomyocytes via mitochondrial pathways. Blood 122:4668–4668. https://doi.org/10.1182/blood.V122.21.4668.4668
Wang GS, Eriksson LC, Xia L, Olsson J, Stal P (1999) Dietary iron overload inhibits carbon tetrachloride-induced promotion in chemical hepatocarcinogenesis: effects on cell proliferation, apoptosis, and antioxidation. J Hepatol 30:689–698. https://doi.org/10.1016/s0168-8278(99)80201-3
Yang F, Li Y, Yan G, Liu T, Feng C, Gong R, Yuan Y et al (2017) Inhibition of iron overload-induced apoptosis and necrosis of bone marrow mesenchymal stem cells by melatonin. Oncotarget. https://doi.org/10.18632/oncotarget.16382
Ke JY, Cen WJ, Zhou XZ, Li YR, Kong WD, Jiang JW (2017) Iron overload induces apoptosis of murine preosteoblast cells via ROS and inhibition of AKT pathway. Oral Dis 23:784–794. https://doi.org/10.1111/odi.12662
Kundson CM, SJ, Korsmeyer (1997) Bcl-2 and Bax function independently to regulate cell death. Nat Genet 16:358–363. https://doi.org/10.1038/ng0897-358
Xiao W, Bin C, Jingyue S, Yu J, Hui Z, Peng Z, Beibei F et al (2018) Iron-induced oxidative stress stimulates osteoclast differentiation via NF-kappaB signaling pathway in mouse model. Metabolism. https://doi.org/10.1016/j.metabol.2018.01.005
Roodman GD (2009) Osteoclasts pump iron. Cell Metab 9:405–406. https://doi.org/10.1016/j.cmet.2009.04.005
Xie W, Lorenz S, Dolder S, Hofstetter W (2016) Extracellular Iron is a modulator of the differentiation of osteoclast lineage cells. Calcif Tissue Int 98:275–283. https://doi.org/10.1007/s00223-015-0087-1
Zhang J, Hu W, Ding C, Yao G, Zhao H, Wu S (2019) Deferoxamine inhibits iron-uptake stimulated osteoclast differentiation by suppressing electron transport chain and MAPKs signaling. Toxicol Lett 313:50–59. https://doi.org/10.1016/j.toxlet.2019.06.007
Zhao S, Kato Y, Zhang Y, Harris S, Ahuja S, Bonewald L (2002) MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res 17:2068–2079. https://doi.org/10.1359/jbmr.2002.17.11.2068
O'Brien CA, Nakashima T, Takayanagi H (2013) Osteocyte control of osteoclastogenesis. Bone 54:258–263. https://doi.org/10.1016/j.bone.2012.08.121
Jia P, Xu YJ, Zhang ZL, Li K, Li B, Zhang W, Yang H (2012) Ferric ion could facilitate osteoclast differentiation and bone resorption through the production of reactive oxygen species. J Orthop Res 30:1843–1852. https://doi.org/10.1002/jor.22133
McCutcheon S, Majeska RJ, Spray DC, Schaffler MB, Vazquez M (2020) Apoptotic osteocytes induce RANKL production in bystanders via purinergic signaling and activation of pannexin channels. J Bone Miner Res. https://doi.org/10.1002/jbmr.3954
Plotkin LI, Gortazar AR, Davis HM, Condon KW, Gabilondo H, Maycas M, Allen MR et al (2015) Inhibition of osteocyte apoptosis prevents the increase in osteocytic receptor activator of nuclear factor kappaB ligand (RANKL) but does not stop bone resorption or the loss of bone induced by unloading. J Biol Chem 290:18934–18942. https://doi.org/10.1074/jbc.M115.642090
Acknowledgement
We would like to thank Yi Lyu in the Key Laboratory for Space Bioscience and Biotechnology for the technical assistance.
Funding
This work sponsored by the Shenzhen Municipal Research Program of Health and Family Planning System (SZXJ2017060), the National Natural Science Foundation of China (51777171), and the Innovation Fund Program for Science and Technology in Longhua District of Shenzhen (2017008).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JY, DD, XL, and JZ. The first draft of the manuscript was written by JY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Jiancheng Yang, Dandan Dong, Xinle Luo, Jianhua Zhou, Peng Shang, and Hao Zhang declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
Animal operation was conducted in accordance with the ethical guidelines for animals of the Medical and Experimental Animal Ethics Committee of Northwestern Polytechnic University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J., Dong, D., Luo, X. et al. Iron Overload-Induced Osteocyte Apoptosis Stimulates Osteoclast Differentiation Through Increasing Osteocytic RANKL Production In Vitro. Calcif Tissue Int 107, 499–509 (2020). https://doi.org/10.1007/s00223-020-00735-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00735-x