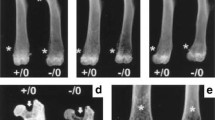
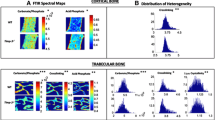
Abstract
The G171V mutation in the low-density lipoprotein receptor-related protein 5 (LRP5) leads to a high bone mass (HBM) phenotype. Studies using HBM transgenic mouse models have consistently found increased bone mass and whole-bone strength, but little attention has been paid to the composition of the bone matrix. The current study sought to determine if the cortical bone matrix composition differs in HBM and wild-type mice and to determine how much of the variance in bone material properties is explained by variance in matrix composition. Consistent with previous studies, HBM mice had greater cortical area, moment of inertia, ultimate force, bending stiffness, and energy to failure than wild-type animals. The increased energy to failure was primarily caused by a large increase in post-yield behavior, with no difference in pre-yield behavior. The HBM mice had increased mineral-to-matrix and collagen cross-link ratios, and decreased crystallinity, carbonate, and acid phosphate substitution as measured by Fourier transform infrared microspectroscopy, but no differences in crystal length, intra-fibular strains, and mineral spacing compared to wild-type controls, as measured by X-ray scattering. The largest between genotype difference in material properties was a twofold increase in the modulus of toughness in HBM mice. Step-wise regression analyses showed that the specific matrix compositional parameters most closely associated with material properties varied between the wild-type and HBM genotypes. Although the mechanisms controlling the paradoxical combination of more mineralized yet tougher bone in HBM mice remain to be fully explained, the findings suggest that LRP5 represents a target to not only build bone mass but also to improve bone quality.




Similar content being viewed by others
References
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Investig 116:1202–1209
Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–523
Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML (2002) A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet 70:11–19
Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP (2002) High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346:1513–1521
Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F (2003) High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 18:960–974
Akhter MP, Fan Z, Rho JY (2004) Bone intrinsic material properties in three inbred mouse strains. Calcif Tissue Int 75:416–420
Padhi D, Jang G, Stouch B, Fang L, Posvar E (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26:19–26
Padhi D, Allison M, Kivitz AJ, Gutierrez MJ, Stouch B, Wang C, Jang G (2013) Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: A randomized, double-blind, placebo-controlled study. J Clin Pharmacol 54:168–178
Recker R, Benson C, Matsumoto T, Bolognese M, Robins D, Alam J, Chiang AY, Hu L, Krege JH, Sowa H, Mitlak B, Myers S (2014) A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res 30:216–224
McColm J, Hu L, Womack T, Tang CC, Chiang AY (2014) Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res 29:935–943
Hernandez CJ, Keaveny TM (2006) A biomechanical perspective on bone quality. Bone 39:1173–1181
Felsenberg D, Boonen S (2005) The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther 27:1–11
Camacho NP, Landis WJ, Boskey AL (1996) Mineral changes in a mouse model of osteogenesis imperfecta detected by Fourier transform infrared microscopy. Connect Tissue Res 35:259–265
Camacho NP, Hou L, Toledano TR, Ilg WA, Brayton CF, Raggio CL, Root L, Boskey AL (1999) The material basis for reduced mechanical properties in oim mice bones. J Bone Miner Res 14:264–272
Fratzl P, Paris O, Klaushofer K, Landis WJ (1996) Bone mineralization in an osteogenesis imperfecta mouse model studied by small-angle x-ray scattering. J Clin Investig 97:396–402
Grabner B, Landis WJ, Roschger P, Rinnerthaler S, Peterlik H, Klaushofer K, Fratzl P (2001) Age- and genotype-dependence of bone material properties in the osteogenesis imperfecta murine model (oim). Bone 29:453–457
Vanleene M, Porter A, Guillot PV, Boyde A, Oyen M, Shefelbine S (2012) Ultra-structural defects cause low bone matrix stiffness despite high mineralization in osteogenesis imperfecta mice. Bone 50:1317–1323
Fratzl P, Roschger P, Eschberger J, Abendroth B, Klaushofer K (1994) Abnormal bone mineralization after fluoride treatment in osteoporosis: a small-angle X-ray-scattering study. J Bone Miner Res 9:1541–1549
Faibish D, Ott SM, Boskey AL (2006) Mineral changes in osteoporosis: a review. Clin Orthop Relat Res 443:28–38
Saito M, Marumo K (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21:195–214
Lopez Franco GE, Blank RD, Akhter MP (2011) Intrinsic material properties of cortical bone. J Bone Miner Metab 29:31–36
Wang X, Shen X, Li X, Agrawal CM (2002) Age-related changes in the collagen network and toughness of bone. Bone 31:1–7
Zimmermann EA, Schaible E, Bale H, Barth HD, Tang SY, Reichert P, Busse B, Alliston T, Ager JW, Ritchie RO 3rd (2011) Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc Natl Acad Sci USA 108:14416–14421
Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Liu Q, Mseeh F, Powell DR, Yang QM, Zambrowicz B, Gerrits H, Gossen JA, He X, Bader M, Williams BO, Warman ML, Robling AG (2011) Lrp5 functions in bone to regulate bone mass. Nat Med 17:684–691
Somerville JM, Aspden RM, Armour KE, Armour KJ, Reid DM (2004) Growth of C57BL/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcif Tissue Int 74:469–475
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486
Donnelly E, Baker SP, Boskey AL, van der Meulen MC (2006) Effects of surface roughness and maximum load on the mechanical properties of cancellous bone measured by nanoindentation. J Biomed Mater Res A 77:426–435
Ross RD, Edwards LH, Acerbo AS, Ominsky MS, Virdi AS, Sena K, Miller LM, Sumner DR (2014) Bone matrix quality following sclerostin antibody treatment. J Bone Miner Res 29:1597–1607
Spevak L, Flach CR, Hunter T, Mendelsohn R, Boskey A (2013) Fourier transform infrared spectroscopic imaging parameters describing acid phosphate substitution in biologic hydroxyapatite. Calcif Tissue Int 92:418–428
Bhatia A, Albazzaz M, Espinoza Orias AA, Inoue N, Miller LM, Acerbo A, George A, Sumner DR (2012) Overexpression of DMP1 accelerates mineralization and alters cortical bone biomechanical properties in vivo. J Mech Behav Biomed Mater 5:1–8
Gallant MA, Brown DM, Hammond M, Wallace JM, Du J, Deymier-Black AC, Almer JD, Stock SR, Allen MR, Burr DB (2014) Bone cell-independent benefits of raloxifene on the skeleton: a novel mechanism for improving bone material properties. Bone 61:191–200
Venkateswarlu K, Chandra Bose A, Rameshbabu N (2010) X-ray peak broadening studies of nanocrystalline hydroxyapatite by Williamson-Hall analysis. Physica B 405:4256–4261
Akhter MP, Wells DJ, Short SJ, Cullen DM, Johnson ML, Haynatzki GR, Babij P, Allen KM, Yaworsky PJ, Bex F, Recker RR (2004) Bone biomechanical properties in LRP5 mutant mice. Bone 35:162–169
Dubrow SA, Hruby PM, Akhter MP (2007) Gender specific LRP5 influences on trabecular bone structure and strength. J Musculoskelet Neuronal Interact 7:166–173
Jacobsen CM, Barber LA, Ayturk UM, Roberts HJ, Deal LE, Schwartz MA, Weis M, Eyre D, Zurakowski D, Robling AG, Warman ML (2014) Targeting the LRP5 pathway improves bone properties in a mouse model of osteogenesis imperfecta. J Bone Miner Res 29:2297–2306
Launey ME, Buehler MJ, Ritchie RO (2010) On the Mechanistic Origins of Toughness in Bone. Annu Rev Mater Res 40:25–53
Nyman JS, Reyes M, Wang X (2005) Effect of ultrastructural changes on the toughness of bone. Micron 36:566–582
Inzana JA, Maher JR, Takahata M, Schwarz EM, Berger AJ, Awad HA (2013) Bone fragility beyond strength and mineral density: Raman spectroscopy predicts femoral fracture toughness in a murine model of rheumatoid arthritis. J Biomech 46:723–730
Bi X, Patil CA, Lynch CC, Pharr GM, Mahadevan-Jansen A, Nyman JS (2011) Raman and mechanical properties correlate at whole bone- and tissue-levels in a genetic mouse model. J Biomech 44:297–303
Wang X, Bank RA, TeKoppele JM, Agrawal CM (2001) The role of collagen in determining bone mechanical properties. J Orthop Res 19:1021–1026
Zioupos P, Currey JD, Hamer AJ (1999) The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res 45:108–116
Viguet-Carrin S, Garnero P, Delmas PD (2006) The role of collagen in bone strength. Osteoporos Int 17:319–336
Ager JW, Nalla RK, Breeden KL, Ritchie RO (2005) Deep-ultraviolet Raman spectroscopy study of the effect of aging on human cortical bone. J Biomed Opt 10:034012
Yerramshetty JS, Akkus O (2008) The associations between mineral crystallinity and the mechanical properties of human cortical bone. Bone 42:476–482
Farlay D, Panczer G, Rey C, Delmas PD, Boivin G (2010) Mineral maturity and crystallinity index are distinct characteristics of bone mineral. J Bone Miner Metab 28:433–445
Jepsen KJ, Hu B, Tommasini SM, Courtland HW, Price C, Terranova CJ, Nadeau JH (2007) Genetic randomization reveals functional relationships among morphologic and tissue-quality traits that contribute to bone strength and fragility. Mamm Genome 18:492–507
Jepsen KJ, Hu B, Tommasini SM, Courtland HW, Price C, Cordova M, Nadeau JH (2009) Phenotypic integration of skeletal traits during growth buffers genetic variants affecting the slenderness of femora in inbred mouse strains. Mamm Genome 20:21–33
Semenov MV, He X (2006) LRP5 mutations linked to high bone mass diseases cause reduced LRP5 binding and inhibition by SOST. J Biol Chem 281:38276–38284
Balemans W, Piters E, Cleiren E, Ai M, Van Wesenbeeck L, Warman ML, Van Hul W (2008) The binding between sclerostin and LRP5 is altered by DKK1 and by high-bone mass LRP5 mutations. Calcif Tissue Int 82:445–453
Ellies DL, Viviano B, McCarthy J, Rey JP, Itasaki N, Saunders S, Krumlauf R (2006) Bone density ligand, sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res 21:1738–1749
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Paszty C, Ke HZ, Simonet WS (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23:860–869
Morvan F, Boulukos K, Clement-Lacroix P, Roman Roman S, Suc-Royer I, Vayssiere B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21:934–945
Hassler N, Roschger A, Gamsjaeger S, Kramer I, Lueger S, van Lierop A, Roschger P, Klaushofer K, Paschalis EP, Kneissel M, Papapoulos S (2014) Sclerostin deficiency is linked to altered bone composition. J Bone Miner Res 29:2144–2151
Acknowledgments
The authors would like to thank Randy Smith for assisting with the collection of FTIRI data, Bonna Holiday for dissecting the HBM tissues, and Alejandro Espinoza Orias for consultation on the analysis of mechanical data. Use of the Rush MicroCT/Histology Core Lab and access to the mechanical testing equipment in the laboratory of Dr. Amarjit Virdi are recognized. Use of the National Synchrotron Light Source and Advanced Photon Source are supported by the U.S. Department of Energy under Contract Nos. DE-AC02-98CH10886 and DE-AC02-06CH1135, respectively.
Funding
This work was supported by NIH Grant R01 AR053949 (MLJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ryan D. Ross, Maleeha Mashiatulla, Alvin S. Acerbo, Jonathan D. Almer, Lisa M. Miller, Mark L. Johnson, and D. Rick Sumner declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ross, R.D., Mashiatulla, M., Acerbo, A.S. et al. HBM Mice Have Altered Bone Matrix Composition and Improved Material Toughness. Calcif Tissue Int 99, 384–395 (2016). https://doi.org/10.1007/s00223-016-0154-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0154-2