Abstract
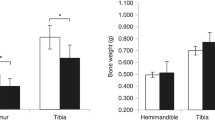
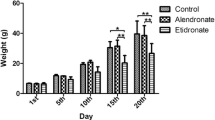
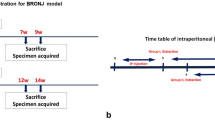
Osteoprotegerin (OPG) is a novel secreted member of the tumor necrosis factor receptor family which plays a crucial role in negative regulation of osteoclastic bone resorption. OPG-deficient (OPG–/–) mice develop severe osteoporosis caused by significant enhancement of bone resorption by osteoclasts. We investigated the effect of administering bisphosphonate on mandibular growth and development in OPG–/– mice. Eight-week-old male OPG–/– mice and wild-type (WT) mice were administered bisphosphonate (1.25 mg/kg body weight) intraperitoneally once every 3 days for 30 days. All bone formation-related parameters and bone resorption-related parameters were significantly lower in OPG–/– mice with bisphosphonate than in those without bisphosphonate. The volume of the whole condyle and the mandibular length in OPG–/– mice without bisphosphonate were significantly smaller than in WT mice without bisphosphonate. Bisphosphonate treatment of the OPG–/– mice resulted in an increase in the volume of the mandibular condyle and mandibular ramus length. In fact, the mandibular ramus length in OPG–/– mice with bisphosphonate was similar to the length in WT mice without bisphosphonate. Histologically, the surface irregularity of the mandibular condyle that was observed in the OPG–/– mice without bisphosphonate tended to be less marked in the OPG–/– mice with bisphosphonate, and the proportion of the area of the cartilage layer relative to the whole condyle was significantly larger in OPG–/– mice with bisphosphonate than in those without bisphosphonate. In conclusion, bisphosphonate treatment results in an increase in mandibular condylar dimensions and normalization of mandibular ramus growth.










Similar content being viewed by others
References
Suda T, Takahasi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand familes. Endocr Rev 20:345–357
Kong YY, Yashida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastgenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13:2412–2424
Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ (2000) RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastgenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA 97:1566–1571
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chan MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS (1998) Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12:1260–1268
Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shima N, Washida N, Tsuda E, Morinaga T, Higashino K, Ozawa H (1998) Severe osteoporosis in mice lacking osteoclastgenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun 247:610–615
Kawata F. Sasaki T (2003) Osteoclast differentiation and characteristic trabecular bone formation during growth plate destruction in osteoprotegerin-deficiency mice. J Electron Microsc 52:515–525
Amizuka N, Shimomura J, Li M, Seki Y, Oda K, Henderson JE, Mizuno A, Ozawa H, Maeda T (2003) Defective bone remodeling in osteoprotegerin-deficiency mice. J Electron Microsc 52:503–513
Whyte MP, Obrecht SE, Finnegan PM, Jones JL, Podgornik MN, McAlister WH, Mumm S (2002) Osteoprotegerin deficiency and juvenile Paget’s disease. N Engl J Med 347:175–184
Cundy T, Hegde M, Naot D, Chong B, King A, Wallace R, Mulley J, Love DR, Seidel J, Fawkner M, Banovic T, Callon KE, Grey AB, Reid IR, Middleton-Hardie CA, Comish J (2002) A mutation in the gene TNFRSF11B encoding osteoprotegerin causes an idiopathic hyperphosphatasia phenotype. Hum Mol Genet 11:2119–2127
Cundy T, Wheadon L, King A (2004) Treatmant of idiopathic hyperphosphatasia with intensive bisphosphonate therapy. J Bone Miner Res 19:703–711
Enlow DH (1982) Handbook of facial growth. 2nd ed. W. B. Saunders, Philadelphia
Antoniades K, Karakasis D, Kapetanos G, Lasaridis N, Tzarou V (1993) Chronic idiopathic hyperphosphatasemia. Oral Surg Oral Med Oral Pathol 76:200–204
Azuma Y, Sato H, Oue Y, Okabe K, Ohta T, Tsuchimoto M, Kiyoki M (1995) Alendronate distributed on bone surfaces inhibits osteoclastic bone resorption in vitro and in experimental hypercalcemia models. Bone 16:235–245
Beresford WA (1975) Schemes of zonation in the mandibular condyle. Am J Ortho 68:189–195
Kimmel DB, Jee WSS (1983) Measurements of area, perimeter and distance: details of data collection in bone histomorphometry. In: Recker RR (eds) Bone histomorphometry. Techniques and interpretation. CRC Press, Boca Raton, FL, pp 89–108
Nakamura M, Udagawa N, Matsuura S, Mogi M, Nakamura H, Horiuchi H, Saito N, Hiraoka BY, Kobayashi Y, Takaoka K, Ozawa H, Miyazawa H, Takahashi N (2003) Osteoprotegerin regulates bone formation through a coupling mechanism with bone resorption. Endocrinology 144:5441–5446
Golob DB, McAlister WH, Mills BG, Fedde KN, Reinus WR, Teitelbaum SL, Beeki S. Whyte MP (1996) Juvenile paget disease: Life-long feature of a mildly affected young woman. J Bone Miner Res 11:132–142
Sasaki T, Kim TW, Debari K, Nagamine H (1996) The replacement processes of growth plate cartilage to bone in endochondral ossification of mandibular condyle. Kaibou-shi 71:106–114
Cole AA, Wezeman FH (1985) Perivascular cell in cartilage canals of the developing mouse epiphysis. Am J Anat 174:119–129
Sakai S (2005) Effect of local administration of cyclophosphamide on mandibular condylar growth in growing rats. Koukubyo Gakkaizassi 72:147–158
Tabuchi M, Miyazawa K, Kimura M, Maeda H, Kawai T, Kameyama Y, Goto S (2005) Enhancement of crude morphogenetic protein-induce new bone formation and normalization of endochondral ossification by bisphosphonate treatment in osteoprotegerin-deficient mice. Calcif Tissue Int 77:239–249
Gennari C, Nuti R, Agnusdei D, Camporeale A, Martini G (1994) Management of oeteoporosis and Paget’s disease. An appraisal of the risks and benefits of drug treatment. Drug Saf 11:176–195
MacGowan JR, Pringle J, Morris VH, Stamp TC (2000) Gross vertebral collapse associated with long-term disodium etidronate treatment for pelvic Paget’s disease. Skeletal Radiol 29:279–282
Schenk R, Eggli P, Fleisch H, Rosini S (1986) Quantitative morphometric evaluation of the inhibitory activity of new aminobisphosphonates on bone resorption in the rat. Calcif Tissue Int 38:342–349
Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF (1995) Bisphosphonates promote apotosis in murine osteoclasts in vitro and vivo. J Bone Miner Res 10:1478–1487
Freisch H (1998) Bisphosphonates: mechanisms of action. Endocr Rev 19:80–100
Smith EJ, McEvoy A, Little DG, Baldock PA, Eisman JA, Gardiner EM (2004) Transient retention of endochondral cartilaginous matrix with bisphosphonate treatment in a long-term rabbit model of distraction osteogenesis. J Bone Miner Res 19:1698–1705
Lehmann HJ, Mouritzen U, Christgau S, Cloos PAC, Christiansen C (2002) Effect of bisphosphonates on cartilage turnover assessed with a newly developed assay for collagen type ? degradation products. Ann Rheum Dis 61:530–533
Evans KD, Lau ST, Oberbauer AM, Martin RB (2003) Alendronate affects long bone length and growth plate morphology in the oim mouse model for Osteogenesis Imperfecta. Bone 32:268–274
Marx RE (2003) Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 61:1115–1117
Marx RE, Sawatani Y, Fortin M, Broumand V (2005) Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 63:1567–1575
Migliorati CA, Schubert MM, Peterson DE, Seneda LM (2005) Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer 104:83–93
Acknowledgments
This study was partially supported by the High-Tech Research Center Project for Aichi Gakuin University, with matching fund subsidy from the Ministry of Education, Culture, Sports, Science and Technology (Japan, 2002-2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kimura, M., Miyazawa, K., Tabuchi, M. et al. Bisphosphonate Treatment Increases the Size of the Mandibular Condyle and Normalizes Growth of the Mandibular Ramus in Osteoprotegerin-Deficient Mice. Calcif Tissue Int 82, 137–147 (2008). https://doi.org/10.1007/s00223-007-9097-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-007-9097-y