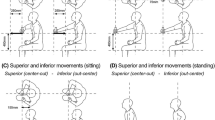
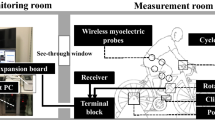
Abstract
Locomotor coordination depends on precise and appropriate adjustments of intra- and interlimb muscle activity phasing. Muscle coordination deficits, in the form of inappropriately phased muscle activity patterns, are well recognized in both the paretic and non-paretic limbs of stroke survivors. Our recent work demonstrated that muscle phasing can be systematically influenced by changing the relative angular positions of limbs in both neurologically intact individuals and people post-stroke. However, it is still unknown whether the observed transient changes in adjusted muscle phasing can be adapted following a short-bout of training on the split-crank ergometer. To explore the extent to which the non-paretic and paretic limbs of people with stroke can adapt to new muscle activity phasing changes, we examined the adaptation of muscle phasing following a short-bout of pedaling training at two specific relative spatial angular positions of limbs that had caused the greatest phasing changes in our previous studies. Twelve individuals with post-cerebral stroke and twelve age- and gender-matched control subjects participated in this study. We demonstrated that both intact and cerebrally impaired nervous systems are capable of adapting new muscle phasing patterns and producing aftereffects that persisted for at least 10 min. However, we observed a completely different trend of aftereffects in post-stroke subjects compared with controls. Specifically, in controls, the aftereffects were observed only in the leg that was in the following position during the adaptation training whereas in post-stroke subjects, aftereffects were observed only in the leg that acted as the leading leg during adaptation, regardless of the limb being paretic or non-paretic. These findings suggest that adapting a new muscle activity pattern during a bilateral locomotor task depends mainly on the relative temporal position of contralateral limb.




Similar content being viewed by others
References
Alibiglou L, Brown DA (2011) Impaired muscle phasing systematically adapts to varied relative angular relationships during locomotion in people post-stroke. J Neurophysiol 105 (Epub ahead of print)
Alibiglou L, Lopez-Ortiz C, Walter CB, Brown DA (2009) Bilateral limb phase relationship and its potential to alter muscle activity phasing during locomotion. J Neurophysiol 102(5):2856–2865
Benecke R, Conrad B, Meinck HM, Hohne J (1983) Electromyographic analysis of bicycling on an ergometer for evaluation of spasticity of lower limbs in man. Adv Neurol 39:1035–1046
Brashers-Krug T, Shadmehr R, Bizzi E (1996) Consolidation in human motor memory. Nature 18; 382(6588):252–255
Brown DA, Kautz SA (1998) Increased workload enhances force output during pedaling exercise in persons with poststroke hemiplegia. Stroke 29(3):598–606
Brown DA, Kautz SA (1999) Speed-dependent reductions of force output in people with poststroke hemiparesis. Phys Ther 79(10):919–930
Brown DA, Kautz SA, Dairaghi CA (1997) Muscle activity adapts to anti-gravity posture during pedalling in persons with post-stroke hemiplegia. Brain 120(Pt 5):825–837
Burridge JH, Wood DE, Taylor PN, McLellan DL (2001) Indices to describe different muscle activation patterns, identified during treadmill walking, in people with spastic drop-foot. Med Eng Phys 23(6):427–434
Cappellini G, Ivanenko YP, Dominici N, Poppele RE, Lacquaniti F (2010) Motor patterns during walking on a slippery walkway. J Neurophysiol 103(2):746–760
Chen H, Hua SE, Smith MA, Lenz FA, Shadmehr R (2006) Effects of human cerebellar thalamus disruption on adaptive control of reaching. Cereb Cortex 16(10):1462–1473
Choi JT, Vining EP, Reisman DS, Bastian AJ (2009) Walking flexibility after hemispherectomy: split-belt treadmill adaptation and feedback control. Brain 132(Pt 3):722–733
Dietz V (1996) Interaction between central programs and afferent input in the control of posture and locomotion. J Biomech 29(7):841–844
Fine MS, Thoroughman KA (2006) Motor adaptation to single force pulses: sensitive to direction but insensitive to within-movement pulse placement and magnitude. J Neurophysiol 96(2):710–720
Fortin K, Blanchette A, McFadyen BJ, Bouyer LJ (2009) Effects of walking in a force field for varying durations on aftereffects and on next day performance. Exp Brain Res 199(2):145–155
Gordon CR, Fletcher WA, Melvill Jones G, Block EW (1995) Adaptive plasticity in the control of locomotor trajectory. Exp Brain Res 102(3):540–545
Hirschberg GG, Nathanson M (1952) Electromyographic recording of muscular activity in normal and spastic gaits. Arch Phys Med Rehabil 33(4):217–225
Jensen L, Prokop T, Dietz V (1998) Adaptational effects during human split-belt walking: influence of afferent input. Exp Brain Res 118(1):126–130
Kautz SA, Brown DA (1998) Relationships between timing of muscle excitation and impaired motor performance during cyclical lower extremity movement in post-stroke hemiplegia. Brain 121(Pt 3):515–526
Krakauer JW, Ghilardi MF, Ghez C (1999) Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci 2(11):1026–1031
Lackner JR, Dizio P (1994) Rapid adaptation to Coriolis force perturbations of arm trajectory. J Neurophysiol 72(1):299–313
Lam T, Anderschitz M, Dietz V (2006) Contribution of feedback and feedforward strategies to locomotor adaptations. J Neurophysiol 95(2):766–773
Layne CS, McDonald PV, Bloomberg JJ (1997) Neuromuscular activation patterns during treadmill walking after space flight. Exp Brain Res 113(1):104–116
Layne CS, Lange GW, Pruett CJ, McDonald PV, Merkle LA, Mulavara AP et al (1998) Adaptation of neuromuscular activation patterns during treadmill walking after long-duration space flight. Acta Astronaut 43(3–6):107–119
Layne CS, Mulavara AP, McDonald PV, Pruett CJ, Kozlovskaya IB, Bloomberg JJ (2001) Effect of long-duration spaceflight on postural control during self-generated perturbations. J Appl Physiol 90(3):997–1006
Milner TE, Hinder MR (2006) Position information but not force information is used in adapting to changes in environmental dynamics. J Neurophysiol 96(2):526–534
Morton SM, Bastian AJ (2006) Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 6; 26(36):9107–9116
Perry J (1993) Determinants of muscle function in the spastic lower extremity. Clin Orthop Relat Res 288:10–26
Prokop T, Berger W, Zijlstra W, Dietz V (1995) Adaptational and learning processes during human split-belt locomotion: interaction between central mechanisms and afferent input. Exp Brain Res 106(3):449–456
Reisman DS, Block HJ, Bastian AJ (2005) Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol 94(4):2403–2415
Reisman DS, Wityk R, Silver K, Bastian AJ (2007) Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 130(Pt 7):1861–1872
Richardson AG, Overduin SA, Valero-Cabre A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E et al (2006) Disruption of primary motor cortex before learning impairs memory of movement dynamics. J Neurosci 29; 26(48):12466–12470
Rogers LM, Brown DA, Gruben KG (2004) Foot force direction control during leg pushes against fixed and moving pedals in persons post-stroke. Gait Posture 19(1):58–68
Scheidt RA, Reinkensmeyer DJ, Conditt MA, Rymer WZ, Mussa-Ivaldi FA (2000) Persistence of motor adaptation during constrained, multi-joint, arm movements. J Neurophysiol 84(2):853–862
Shadmehr R, Mussa-Ivaldi FA (1994) Adaptive representation of dynamics during learning of a motor task. J Neurosci 14(5 Pt 2):3208–3224
Shiavi R, Bugle HJ, Limbird T (1987) Electromyographic gait assessment, part 2: preliminary assessment of hemiparetic synergy patterns. J Rehabil Res Dev 24(2):24–30
Thoroughman KA, Shadmehr R (1999) Electromyographic correlates of learning an internal model of reaching movements. J Neurosci 1; 19(19):8573–8588
Vasudevan EV, Bastian AJ (2010) Split-belt treadmill adaptation shows different functional networks for fast and slow human walking. J Neurophysiol 103(1):183–191
Weber KD, Fletcher WA, Gordon CR, Melvill Jones G, Block EW (1998) Motor learning in the “podokinetic” system and its role in spatial orientation during locomotion. Exp Brain Res 120(3):377–385
Zijlstra W, Dietz V (1995) Adaptability of the human stride cycle during split-belt locomotion walking. Gait Posture 3:250–257
Acknowledgments
We would like to thank Chrystal L. Johnston for her kind assistance in recruitment of subjects, Jamie K. Burgess and Sam Perlmutter for their invaluable help in creating and developing the kinetic analysis program. This work was supported, in part, by American Heart Association Grant 0715536Z.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alibiglou, L., Brown, D.A. Relative temporal leading or following position of the contralateral limb generates different aftereffects in muscle phasing following adaptation training post-stroke. Exp Brain Res 211, 37–50 (2011). https://doi.org/10.1007/s00221-011-2644-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2644-9