Abstract
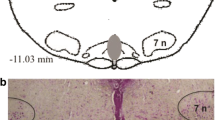
Exposure to acute intermittent hypoxia (AIH) evokes persistent increase in respiratory activity that lasts up to 60 min after hypoxic episodes have ceased. This persistent increase in phrenic nerve activity (PNA) is known as phrenic long-term facilitation (LTF). AIH-induced phrenic LTF in anesthetized rats is serotonin dependant. The present study was performed to determine whether microinjection of methysergide (4 mM, 20 ± 5 nl), a broad spectrum 5-HT receptor antagonist, into the caudal raphe nuclei influences phrenic LTF. Peak integrated PNA and respiratory frequency were recorded at 15, 30, and 60 min after five 3-min episodes of normocapnic hypoxia in urethane-anesthetized, vagotomized, paralyzed and ventilated male Sprague–Dawley rats. In control animals, phrenic nerve amplitude was elevated 66.7 ± 8.6% from baseline 1 h after episodic hypoxia, indicating phrenic LTF. Experimental microinjections of methysergide prior to AIH exposure attenuated phrenic LTF (amplitude increase 2.62 ± 2.9% over baseline). We conclude that methysergide microinjections into the caudal raphe region attenuated phrenic LTF induced by AIH, indicating involvement of 5-HT receptor activation at a supraspinal level.






Similar content being viewed by others
References
Bach KB, Mitchell GS (1996) Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104:251–260
Baker TL, Mitchell GS (2000) Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529(Pt 1):215–219
Baker-Herman TL, Mitchell GS (2002) Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22:6239–6246
Baker-Herman TL, Mitchell GS (2008) Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol 162:8–17
Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38:1083–1152
Cao Y, Fujito Y, Matsuyama K, Aoki M (2006a) Effects of electrical stimulation of the medullary raphe nuclei on respiratory movement in rats. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192:497–505
Cao Y, Matsuyama K, Fujito Y, Aoki M (2006b) Involvement of medullary GABAergic and serotonergic raphe neurons in respiratory control: electrophysiological and immunohistochemical studies in rats. Neurosci Res 56:322–331
Carev M, Valic M, Pecotic R, Karanovic N, Valic Z, Pavlinac I, Dogas Z (2009) Propofol abolished the phrenic long term facilitation in rats. Respir Physiol Neurobiol. doi:10.1016/j.resp.2009.12.011
Dobbins EG, Feldman JL (1994) Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347:64–86
Erickson JT, Millhorn DE (1994) Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol 348:161–182
Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS (2000) Long term facilitation of phrenic motor output. Respir Physiol 121:135–146
Fuller DD, Zabka AG, Baker TL, Mitchell GS (2001) Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol 90:2001–2006 (discussion 2000)
Haji A, Takeda R, Okazaki M (2000) Neuropharmacology of control of respiratory rhythm and pattern in mature mammals. Pharmacol Ther 86:277–304
Helke CJ, Capuano S, Tran N, Zhuo H (1997) Immunocytochemical studies of the 5-HT(1A) receptor in ventral medullaryneurons that project to the intermediolateral cell column and contain serotonin or tyrosine hydroxylase immunoreactivity. J Comp Neurol 379:261–270
Hodges MR, Richerson GB (2008) Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol 164:222–232
Holtman JR Jr, Norman WP, Gillis RA (1984) Projections from the raphe nuclei to the phrenic motor nucleus in the cat. Neurosci Lett 44:105–111
Holtman JR Jr, Dick TE, Berger AJ (1987) Serotonin-mediated excitation of recurrent laryngeal and phrenic motoneurons evoked by stimulation of the raphe obscurus. Brain Res 417:12–20
Kachidian P, Poulat P, Marlier L, Privat A (1991) Immunohistochemical evidence for the coexistence of substance P, thyrotropin-releasing hormone, GABA, methionine-enkephalin, and leucin-enkephalin in the serotonergic neurons of the caudal raphe nuclei: a dual labeling in the rat. J Neurosci Res 30:521–530
Kinkead R, Mitchell GS (1999) Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. Am J Physiol 277:R658–R666
Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS (2001) Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp Biochem Physiol A Mol Integr Physiol 130:207–218
Lalley PM (1986) Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. J Physiol 380:373–385
Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB Jr, Mitchell GS (2001) Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21:5381–5388
MacFarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS (2008) Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol 164:263–271
Mahamed S, Mitchell GS (2008) Simulated apnoeas induce serotonin-dependent respiratory long-term facilitation in rats. J Physiol 586:2171–2181
Millhorn DE (1986) Stimulation of raphe (obscurus) nucleus causes long-term potentiation of phrenic nerve activity in cat. J Physiol 381:169–179
Millhorn DE, Eldridge FL, Waldrop TG (1980) Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol 42:171–188
Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB Jr (2001) Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol 90:2466–2475
Morris KF, Arata A, Shannon R, Lindsey BG (1996) Long-term facilitation of phrenic nerve activity in cats: responses and short time scale correlations of medullary neurones. J Physiol 490(Pt 2):463–480
Muneoka KT, Takigawa M (2003) 5-Hydroxytryptamine7 (5-HT7) receptor immunoreactivity-positive ‘stigmoid body’-like structure in developing rat brains. Int J Dev Neurosci 21:133–143
Nucci TB, Branco LG, Gargaglioni LH (2008) 5-HT1A, but not 5-HT2 and 5-HT7, receptors in the nucleus raphe magnus modulate hypoxia-induced hyperpnoea. Acta Physiol (Oxf) 193:403–414
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Elevier Academic Press, San Diego
Rea MA, Aprison MH, Felten DL (1982) Catecholamines and serotonin in the caudal medulla of the rat: combined neurochemical-histofluorescence study. Brain Res Bull 9:227–236
Richter DW, Manzke T, Wilken B, Ponimaskin E (2003) Serotonin receptors: guardians of stable breathing. Trends Mol Med 9:542–548
Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C (1997) Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol 388:169–190
Acknowledgments
The authors thank Jelena Baricevic for her technical assistance; professor Ivica Grkovic from the Department of Anatomy, Histology and Embryology, University of Split School of Medicine, for his histological expertise; and Dr. JoAnn A Giaconi (Glaucoma Division, Jules Stein Eye Institute, David Geffen School of Medicine, UCLA, Los Angeles, USA) for the language correction of the manuscript. This work has been supported by the Croatian Ministry of Science, Education and Sport Grants 216-2163166-3342 and 216-2163166-0513.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valic, M., Pecotic, R., Pavlinac, I. et al. Microinjection of methysergide into the raphe nucleus attenuated phrenic long-term facilitation in rats. Exp Brain Res 202, 583–589 (2010). https://doi.org/10.1007/s00221-010-2161-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2161-2