Abstract
Ellagitannins are known to possess many beneficial and health-promoting properties, including antioxidant and antimicrobial effects, arising from the activity of both native compounds and the products of their degradation or metabolism. The wide range of beneficial properties is attributable to the great structural variety of these compounds, even though all of them belong to the same polyphenolic group, namely hydrolyzable tannins. Therefore, the potential of individual ellagitannins must be studied separately with the view to their application, as natural substances, in medicine or the food industry. The objective of the present work was to elucidate the effects of temperature and medium pH on the stability of the two main raspberry ellagitannins, i.e., lambertianin C and sanguiin H-6, in aqueous solutions. Experiments were conducted within the temperature range of 20–80 °C and pH 2–8 over 0–24 h of incubation. The content of the studied ellagitannins and the products of their decomposition was investigated using HPLC-DAD and LC–MS, respectively, with an Orbitrap detector. It has been found that the studied ellagitannins are stable in acidic conditions, but are rapidly degraded in neutral and mildly basic media at elevated temperature (60–80 °C). In mildly acidic conditions (pH 6) ellagitannins hydrolyze to intermediate products, that is, sanguiin H-10 isomers, sanguiin H-2, and galloyl-HHDP-glucose isomers, with the main end products being ellagic and gallic acids. In addition to hydrolysis, ellagitannins may also undergo oxidation to compounds containing a dehydrohexahydroxydiphenoyl (DHHDP) group, also accompanied by the presence of brevifolin carboxylic acid, a product of DHHDP hydrolysis.
Similar content being viewed by others
Introduction
Ellagitannins, which can be defined as polyphenols belonging to the group of hydrolyzable tannins, are naturally found in many plants and are known to possess a range of beneficial and health-promoting properties, including antioxidant, anti-inflammatory, and prebiotic activities [1,2,3]. These compounds are usually ingested by humans by the consumption of berries, such as strawberries, raspberries, and blackberries, both raw and processed. Ellagitannins are also found in walnuts, pomegranates, herbs, and, to a lesser extent, wines aged in oak barrels [2, 4, 5]. The daily consumption of ellagitannins as ellagic acid is estimated at 4–12 mg/day, although data on this subject are scarce [2]. In chemical terms, ellagitannins are esters of hexahydroxydiphenic acid (HHDP) and saccharides (usually glucose). They occur in many forms since saccharide molecules may bind HHDP and gallic acid residues in different combinations, also creating oligomers [6]. It is generally acknowledged that ellagitannins are hydrolyzed by acids and bases, as a result of which HHDP residues split from the molecules and are transformed to ellagic acid. While this is true, this description is not very precise, as according to some reports in basic conditions ellagitannins may undergo numerous transformations resulting from hydrolysis, deprotonation, and oxidation, while in acidic media they remain relatively stable [7]. The best case in point is the natural fruit environment, which does not degrade ellagitannins, in contrast to high concentrations of mineral acids or trifluoroacetic acid, which are used for ellagitannin hydrolysis in analytical methods [8]. According to Appel [9], phenolic compounds undergo the most extensive transformations at pH > 8, where they assume ionic form (phenolate ions) or are converted to quinones as a result of oxidation, and are characterized by high reactivity conducive to degradation. Furthermore, another important factor crucial to ellagitannin stability is molecular structure. According to Tanaka et al. [10], ellagitannins are more readily soluble in water than gallotannins due to the HHDP moieties contained in the former. The hydroxyl groups in gallotannins form more hydrophobic and hydrogen bonds between molecules, which makes them more stable and less susceptible to degradation. Another major factor affecting polyphenolic stability is temperature. It is generally known that high temperature applied during technological processes or storage is conducive to polyphenolic degradation. Temperature significantly influences the stability of these compounds in fruits and fruit products characterized by low pH, especially during storage. As reported by Qu et al. [11], ellagitannins such as punicalagin A and B obtained from pomegranate peel were degraded by more than 30% at pH 3.5 and 37 °C after 180 days. In the case of basic pH conditions, temperature is less important. In a study of Tuominen and Sundman [7], ellagitannins incubated at ambient temperature exhibited half-lives of less than 10 min at pH 10. The above results indicate that ellagitannin degradation mostly depends on pH conditions. Due to the susceptibility of these compounds to hydrolysis at high pH, they are readily degraded in the human gastrointestinal system to ellagic acid, which is subsequently metabolized to urolithins [4, 12]. Among the polyphenols present in raspberries, the largest group consists of ellagitannins, which account for 53–76% of the total, depending on the plant cultivar [13]. In turn, lambertianin C and sanguiin H-6 account for approx. 80% of total raspberry ellagitannins on average [14]. These compounds are trimers and dimers, respectively, of galloyl-bis-HHDP-glucose, a monomer in which the anomeric carbon C1 is bound to a gallic acid residue at position α or β (forming potentillin and casuarictin, accordingly). The oligomerization of monomeric units occurs via oxidative C–O coupling, in which the oxygen is donated by the hydroxyl group of the gallic acid residue of the first monomer, forming an ether bond with the HHDP residue of the other monomer [6].
Given the absence of literature data on the subject, the aim of this study was to determine the effects of parameters such as pH, temperature, and incubation time on the stability of the main raspberry ellagitannins (lambertianin C and sanguiin H-6) in aqueous solutions. Furthermore, the products of the degradation of these ellagitannins were identified.
Materials and methods
Plant material
The study material was raspberry ellagitannin preparation (REP), which contained 44% lambertianin C and 35% sanguiin H-6. The full method of REP production was detailed in our previous publication [15]. Briefly, raspberry pomace from juice manufacture was extracted with 60% acetone. Following acetone removal, the extract was injected onto a XAD 1600 N column (DOW, Midland, MI, USA) packed with Amberlite resin. The phenolic compounds retained in the column were eluted with ethanol at concentrations ranging from 10 to 60%, obtaining fractions with different qualitative composition. Ellagitannins were obtained in the fraction eluted with 40% ethanol. This fraction was then subjected to alcohol removal by means of a rotary vacuum evaporator and freeze-dried to obtain REP, which was used in stability studies. The ellagitannin composition of REP is given in Table 1.
Chemicals and standards
Citric acid monohydrate and disodium phosphate for buffer preparation were obtained from POCH (Gliwice, Poland). For ellagitannin analysis, all solvents were HPLC or LC–MS grade. Acetonitrile and formic acid were purchased from Sigma-Aldrich Chemie (Steinheim, Germany), and orthophosphoric acid was from Avantor Performance Materials B.V. (Deventer, Holland). Lambertianin C and sanguiin H-6 standards were prepared in our laboratory according to the procedure described in a previous work [13]. Ultrapure water for buffer preparation and HPLC and LC–MS analysis was obtained from a Hydrolab HLP5 System (Straszyn, Poland).
Stability studies of lambertianin C and sanguiin H-6
Stability studies were conducted in pH 2, 4, 6, 7, and 8 buffers, which were obtained by mixing 0.1 M citric acid and 0.2 M disodium phosphate solutions at the following proportions (v/v at 20 °C): 3/0, 2/1.5, 1.1/2.4, 0.9/2.6, and 0.065/3, respectively. The pH of each solution was tested using a pH-meter. In the case of higher process temperatures, the proportions of the solutions were modified accordingly to ensure a desired pH value. Stability evaluation was performed for an ellagitannin solution with a concentration of approx. 200 mg/L, which corresponds to that of raspberry juice [13]. For this purpose, 20 mg of REP was weighed into a glass vessel, and 100 mL of a buffer solution with an appropriate pH was added. After the dissolution of the preparation, the sealed vessel was placed in an incubator. Incubation was conducted in four temperature variants, i.e., 20 °C, 40 °C, 60 °C, and 80 °C, for 24 h. Samples for analysis were taken at the following incubation times: 0 h (immediately after REP dissolution), 1 h, 2 h, 4 h, 8 h, and 24 h. The samples were immediately diluted 1:1 (v/v) with 0.1 M citric acid solution to stabilize the ellagitannins, centrifuged at 10,000×g, and transferred to chromatographic vials.
Quantitation of ellagitannins
The content of ellagitannins in incubated samples was determined according to procedure described previously by Sójka et al. [13]. Analysis were carried out using Smartline chromatograph (Knauer, Berlin, Germany) composed of a degasser (Manager 5000), two pumps (P1000), autosampler (3950), thermostat, and PDA detector (2800). Lambertianin C and sanguiin H-6 were separated on Gemini C18 110 Å, 250 mm × 4.6 mm i.d., 5 µm, column (Phenomenex, Torrance, CA, USA). The mobile phase consisted of solvent A (0.05% (v/v) phosphoric acid–water) and solvent B [83:17 (v/v) acetonitrile–water with 0.05% phosphoric acid]. The column temperature was set to 35 °C, the flow rate was 1.25 mL/min, and the gradient program was as follows: 0–5 min, 5% (v/v) B; 5–10 min, 5–15% (v/v) B; 10–35 min, 15–40% (v/v) B; 35–40 min, 40–73% (v/v) B; 40–44 min, 73% (v/v) B; 44–46 min, 73–5% (v/v) B; and 46–54 min, 5% (v/v) B. The injection volume was 20 µL. Data were collected using ClarityChrom v.3.0.5.505 software (Knauer, Berlin, Germany). Both compounds were detected at 250 nm, and standard curves were used for quantitation.
Identification of degradation products
The degradation products of lambertianin C and sanguiin H-6 were identified using LC–MS for the variant in which REP was incubated at pH 6 and 80 °C. This variant was selected due to the fact that it was found to have the highest concentration of degradation products. Identification was carried out with the use of a Dionex Ultimate 3000 high performance liquid chromatograph (HPLC) coupled with a DAD and Q Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The mobile phase consisted of 1% (v/v) formic acid in water (solvent A) and 80:20 (v/v) acetonitrile–water (solvent B). The following gradient was used: 0–6.5 min, 5% (v/v) B; 6.5–12.5 min, 5–15% (v/v) B; 12.5–44 min, 15–45% (v/v) B; 44–45 min, 45–75% (v/v) B; 45–50 min, 75% (v/v) B; 50–52 min, 75–5% (v/v) B; and 52–65 min, 5% (v/v) B. The column used was a 250 mm × 4.6 mm i.d., 5 µm, Luna C18(2) 100 Å (Phenomenex, Torrance, CA). The column temperature was set to 35 °C, the flow rate was 1 mL/min, and the injection volume was 20 µL. Chromatographic data were collected using Xcalibur software (Thermo). The MS system coupled to the HPLC was an Orbitrap mass spectrometer equipped with an H-ESI probe used in the negative mode. The source parameters were as follows: vaporizer temperature of 500 °C, ion spray voltage of 4 kV, capillary temperature of 400 °C; and sheath gas and auxiliary gas flow rates of 75 and 20 units, respectively. The detector was operated in either the full MS or full MS/dd-MS2 scan modes. In the full MS mode, the scan range of m/z 200–2000 was used. To generate MS2 data, the full MS/dd-MS2 scan mode was applied. The collision energy used to generate MS2 spectra was set to 20. Tuning and optimization were performed using direct injection of REP diluted in an 80:20 (v/v) mixture of mobile phases A and B at a flow rate of 0.25 mL/min.
Statistics
Sanguiin H-6 and lambertianin C half-lives were analyzed using the t test. To examine the course of ellagitannin hydrolysis, the peak areas of degradation products acquired in the selected ion monitoring (SIM) mode by the mass detector were analyzed using k-means clustering. Prior to analysis, the data obtained from the detector were standardized. Statistical analysis was conducted using Statistica v.10 software (StatSoft, Tulsa, OK).
Results and Discussion
Kinetics of ellagitannin degradation
Table 2 shows the obtained degradation rate constants (k) and half-lives (t½) for sanguiin H-6 and lambertianin C. These values were calculated for conditions in which a decrease in ellagitannins was observed on the basis of fitting a first-order kinetic model to experimental data. Since in acidic media (pH 2 and 4) at temperatures in the range of 20–60 °C the studied ellagitannins exhibited high stability, this kinetic model could not be used for 24 h incubation.
Analysis indicated that both parameters, that is, pH conditions and temperature, significantly affected the degradation rate of the examined ellagitannins, with the crucial factor being basic pH (Figs. 1, 2). At pH 8, at all the temperature levels applied, both lambertianin C and sanguiin H-6 underwent considerable degradation already during the first 2 h of incubation. At 20 °C, the half-lives of the two compounds amounted to approx. 2.5 h, while at 80 °C their values fell to less than 0.5 h. A decrease in pH to 7 inhibited degradation, especially at 20 °C; at higher temperatures degradation processes decreased but did not cease completely. A further decline in pH led to greater stability of the studied ellagitannins. In mildly acidic conditions at ambient temperature, they were not degraded over 24 h.
Nevertheless, degradation did occur at higher temperatures with the half-lives of both compounds at 60 °C and 80 °C decreasing to less than 2.5 h. In the case of pH in the range of 2–4, that is, in natural conditions for ellagitannins, the studied compounds exhibited high stability, especially at 20–60 °C. At 80 °C, they were gradually degraded only after 4 h of incubation. It should also be noted that at pH 2 and 80 °C the half-lives of both compounds exceeded 60 h. Similar results for raspberry ellagitannin hydrolysis were obtained by Daniel et al. [16], who observed a pronounced increase in ellagic acid concentration in neutral (pH 7) and basic (pH 8) raspberry extract solutions. The same authors also reported high stability of raspberry ellagitannins in acidic conditions (pH 2).
The ellagitannins investigated in this work revealed lower stability as compared to those examined by Tuominen and Sundman [7], who studied monomeric ellagitannins with lower molecular weight (digalloyl-4,6-HHDP-glucose, pedunculagin, and geraniin) consisting of one glucose molecule linked to HHDP, dehydrohexahydroxydiphenoyl (DHHDP), and/or gallic acid residues. Those compounds exhibited relatively high stability at pH 8 at ambient temperature, with a degradation rate constant of less than 0.1 in those conditions. In our study, the reaction rate constants obtained in the same conditions (pH 8 and 20 °C) for sanguiin H-6 and lambertianin C were 0.77 and 0.75, respectively, and markedly increased at higher incubation temperatures. At pH 8, for both compounds a temperature increase from 20 °C to 40 °C or 60 °C on average doubled the parameter k, while an increase from 20 °C to 80 °C led to an approx. sixfold increment in that parameter. A significant effect of temperature on the stability of monomeric ellagitannins was reported by Hemingway and Hillis [17]. In their study, at pH 8 and 0 °C bis-HHDP-glucose did not exhibit any degradation after 3 h of incubation, while a temperature increase to 50 °C caused more than 80% degradation.
A comparison of the half-lives and degradation rate constants obtained in this study provides mixed results in terms of determining the ellagitannin with superior stability parameters. The t test (Table 2) for the half-lives of sanguiin H-6 and lambertianin C did not reveal significant differences in four experimental variants, with lambertianin C exhibiting higher stability in seven variants and sanguiin H-6 in two variants. It should also be noted that in many cases the half-lives of these compounds were similar, with the differences possibly attributable to the calculations in the applied kinetic model.
Nevertheless, the presented data indicate that both of the studied dimeric and trimeric ellagitannins are less stable than monomeric ellagitannins and gallotannins. The study by Tuominen and Sundman [7] showed that monomeric ellagitannins, such as digalloyl-4,6-HHDP-glucose and pedunculagin, degraded in only approx. 10% after 6 h of incubation at pH 8 at ambient temperature. Thus, it may be inferred that larger structures and higher molecular weights impair ellagitannin stability in basic conditions. According to Tanaka [10] and Tuominen and Sundman [7], the high stability of gallotannins is attributable to the greater number of hydrophobic and hydrogen bonds between their molecules in aqueous solutions, which in effect protects OH groups from deprotonation and further decomposition. In the case of ellagitannins, the presence of HHDP groups facilitates their solubility in water, thus increasing their susceptibility to hydrolysis.
Degradation products of sanguiin H-6 and lambertianin C
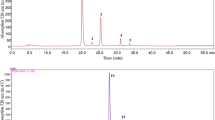
Qualitative analysis (LC–MS) was performed for samples incubated at 80 °C and pH 6 for 0–24 h. These conditions were selected because in quantitative analysis they had shown the greatest number of peaks arising from the degradation of native ellagitannins (Fig. 3). Table 3 presents the identification of 26 compounds found in REP before and after incubation. Native ellagitannins predominantly underwent hydrolysis to intermediate products, the most abundant of which were sanguiin H-10 isomer, sanguiin H-2, and galloyl-bis-HHDP-glucose isomers. The main end products of hydrolysis were ellagic and gallic acids (Fig. 4). In addition, we detected some products that may have been generated by oxidation, but their amount was much lower.
Chromatogram (250 nm) of ellagitannins contained in REP and their degradation products for different incubation times at pH 6 and 80 °C. Peak numbers correspond to those in Table 3
The incubated material, that is, REP, which is a highly concentrated ellagitannin preparation, contains nine ellagitannins, with the most abundant ones being lambertianin C (peak 19) and sanguiin H-6 (peak 23). The remaining seven constituents of REP, occurring in much lower amounts, are: two isomers of sanguiin H-10 (peaks 9 and 16), lambertianin C derivatives without an ellagic moiety (peaks 11 and 20), sanguiin H-10 without an ellagic moiety (peak 17), lambertianin C without HHDP-HHDP-glucose (peak 18), and galloyl-bis-HHDP-glucose (peak 22). Other compounds were detected only after incubation as they resulted from the hydrolysis or oxidation of the native ellagitannins.
Table 3 presents the responses of the mass detector to the content of the various compounds at different incubation times. These data show how their concentrations changed in the course of the process. To examine the hydrolysis of the studied ellagitannins, the data from the detector were analyzed using k-means cluster analysis (Fig. 5). The studied compounds have been grouped in 4 clusters. The first one consists of compounds which were degraded in the first stage of hydrolysis (peaks 9, 11, 19, and 23), that is, native ellagitannins. Their concentrations did not increase with incubation time.
The second and third clusters consist of intermediate products of early (1–2 h) and late hydrolysis (4–8 h). The second cluster contains compounds that arose due to the removal of an HHDP residue from sanguiin H-6 leading to sanguiin H-10 isomers (peaks 16 and 21) as well as the removal of HHDP and HHDP-glucose residues (in that order) from lambertianin C leading to lambertianin C without an ellagic moiety (peak 20) and lambertianin C without bis-HHDP-glucose (peak 18, respectively). Another member of cluster 2 is galloyl-bis-HHDP-glucose (peak 22) resulting from the removal of this moiety from either lambertianin C or sanguiin H-6. The third cluster consists of compounds arising from further decomposition of those from cluster 2, that is, sanguiin H-10 without an ellagic moiety (peak 17), sanguiin H-2 (peak 24) created by the removal of HHDP-glucose from sanguiin H-10 isomers, and 3 isomers of galloyl-HHDP-glucose (peaks 6–8). The galloyl-HHDP-glucose isomers generated at this stage of hydrolysis may occur in the form of anomers. In addition, cluster 3 reveals some compounds which may have arisen due to oxidation, such as galloyl-bis-HHDP-glucose (peak 14) producing pseudomolecular ion m/z 933 resulting from deprotonation and then oxidation to a semiquinone [7, 9]. Other 3 oxidation products may be represented by peaks 10, 12, and 13. They gave molecular ion m/z 967 and a characteristic sequence of fragment ions: m/z 923, 879, and 851, resulting from the removal of COOH (m/z 44) and C = OCOOH (m/z 72) groups. According to Tuominen and Sundman [7], these structures are attributable to further oxidation of quinones. Finally, cluster 3 features a compound (peak 4) defined as a geraniin-type ellagitannin, producing pseudomolecular ion m/z 951. This compound is characterized by the presence of a DHHDP group resulting from the oxidation of a HHDP group. This is corroborated by the presence of brevifolin carboxylic acid (peak 15) in the hydrolysate, as this acid arises from hydrolysis of DHHDP [7, 24].
Finally, the fourth cluster consists of the end products of ellagitannin decomposition, including gallic acid (peak 1), galloyl-glucose (peak 2), galloyl-HHDP-glucose isomer (peak 3), brevifolin carboxylic acid (peak 15), ellagic acid (peak 25), and sanguisorbic acid dilactone (peak 26). All of them, except for brevifolin carboxylic acid, result from hydrolysis of native ellagitannins. The detected sanguisorbic acid dilactone indicates the presence of oligomeric ellagitannins—in this case lambertianin C and sanguiin H-6 [10]. This cluster also reveals a compound that could not be identified (peak 3), characterized by light absorption at 360 nm and pseudomolecular ion m/z 439. Given its specific absorption properties, it may be a degradation product of an oxidized ellagitannin [7]. Three of the aforementioned compounds (gallic acid, ellagic acid, and sanguisorbic acid dilactone) were also identified by García-Villalba et al. [20], who conducted 24 h acidic hydrolysis of pomegranate extracts. Similarly as in the present study, those compounds were generated by the hydrolysis of sanguiin H-10 and other native pomegranate ellagitannins.
The present study shows that the two main raspberry ellagitannins, that is, trimeric lambertianin C and dimeric sanguiin H-6 are stable in acidic conditions, but undergo rapid degradation in neutral and mildly basic conditions at elevated temperature (60–80 °C). It has been shown that in mildly acidic conditions (pH 6) they hydrolyze, yielding intermediate products (mostly sanguiin H-10 isomer, sanguiin H2, and galloyl-HHDP-glucose isomers), with the end products being ellagic and gallic acids. In addition to hydrolysis, the studied ellagitannins also undergo oxidation processes giving rise to molecules containing a DHHDP group, accompanied by its hydrolysis product, namely, brevifolin carboxylic acid.
Knowledge of these properties is crucial not only to raspberry ellagitannin applications in the food and pharmaceutical industries, but also in terms of the effects of neutral and basic conditions and high temperatures in biological studies of these compounds.
References
Chung K-T, Wei C-I, Johnson MG (1998) Are tannins a double-edged sword in biology and health? Trends Food Sci Technol 9:168–175
Landete JM (2011) Ellagitannins, ellagic acid and their derived metabolites: a review source, metabolism, function and health. Food Res Int 44:1150–1160
Bialonska D, Kasimsetty SG, Schrader KK, Ferreira D (2009) The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on growth on human gut bacteria. J Agric Food Chem 57:8344–8349
Lipińska L, Klewicka E, Sójka M (2014) Structure, occurrence and biological activity of ellagitannins: a general review. Acta Sci Pol Technol Aliment 13(3):289–299
Maier M, Oelbermann A-L, Renner M, Weidner E (2017) Screening of European medicinal herbs on their tannin content–new potential tanning agents for the leather industry. Ind Crops Prod 99:19–26
Okuda T, Yoshida T, Hatano T, Ito H (2009) In: Quideau S (ed) Ellagitannins renewed the concept of tannins. World Scientific Publishing, Singapore, 1:1–55
Tuominen A, Sundman T (2013) Stability and oxidation products of hydrolysable tannins in basic conditions detected by HPLC/DAD-ESI/QTOF/MS. Phytochem Anal 24:424–435
Klimczak E, Król B (2010) Determination of different forms of ellagic acid in by-products from strawberry processing. Zywn Technol Jakosc 4(71):81–94
Appel HM (1993) Phenolics in ecological interactions: the importance of oxidation. J Chem Ecol 19(7):1521–1552
Tanaka T (2009) In: Quideau S (ed) Ellagitannins renewed the concept of tannins. World Scientific Publishing, Singapore, Ch. 4:119–151
Qu W, Li P, Hong J, Liu Z, Chen Y, Breksa AP III, Pan Z (2014) Thermal stability of liquid antioxidative extracts from pomegranate peel. J Sci Food Agric 94:1005–1012
Piwowarski JP, Granica S, Stefańska J, Kiss AK (2016) Differences in metabolism of ellagitannins by human gut microbiota ex vivo cultures. J Nat Prod 79:3022–3030
Sójka M, Macierzyński J, Zaweracz W, Buczek M (2016) Transfer and mass balance of ellagitannins, anthocyanins, flavan-3-ols, and flavonols during the processing of red raspberries (Rubus idaeus L.) to juice. J Agric Food Chem 64:5549–5563
Gasperotti M, Masuero D, Vrhovsek U, Guella G, Mattivi F (2010) Profiling and accurate quantification of Rubus ellagitannins and ellagic acid conjugates using direct UPLC-Q-TOF HDMS and HPLC-DAD analysis. J Agric Food Chem 58:4602–4616
Klewicka E, Sójka M, Klewicki R, Kołodziejczyk K, Lipińska L, Nowak A (2016) Ellagitannins from raspberry (Rubus idaeus L.) fruit as natural inhibitors of Geotrichum candidum. Molecules 21:908
Daniel EM, Ratnayake S, Kinstle T, Stronger GD (1991) The effects of pH and rat intestinal contents on the liberation of ellagic acid from purified and crude ellagitannins. J Nat Prod 54:4:946–952
Hemingway RW, Hillis WE (1971) Behavior of ellagitannins, gallic acid, and ellagic acid under alkaline conditions. Tappi J 54:933–936
Álvarez-Fernádez MA, Hornedo-Ortega R, Cerezo AB, Troncoso AM, García-Parrilla MC (2016) Determination of nonanthocyanin phenolic compounds using high-resolution mass spectrometry (UHPLC-Orbitrap-MS/MS) and impact of storage conditions in a beverage made from strawberry by fermentation. J Agric Food Chem 64:1367–1376
Dincheva I, Badjakov I, Kondakova V, Dobson P, McDougall G, Stewart D (2013) Identification of the phenolic components in Bulgarian raspberry cultivars by LC-ESI-MSn. Int J Agric Sci Res 3(3):127–138
García-Villalba R, Espín JC, Aaby K, Alsalvar C, Heinonen M, Jacobs G, Voorspoels S, Koivumäki T, Kroon PA, Pelvan E, Saha S, Tomás-Barberán FA (2015) Validated method for the characterization and quantification of extractable and nonextractable ellagitannins after acid hydrolysis in pomegranate fruits, juices, and extracts. J Agric Food Chem 63:6555–6566
Kähkönen M, Kylli P, Ollilainen V, Salminen J-P, Heinonen M (2012) Antioxidant activity of isolated ellagitannins from red raspberries and cloudberries. J Agric Food Chem 60:1167–1174
Kajdžanowska M, Gjamovski V, Stefova M (2010) HPLC-DAD-ESI-MSn identification of phenolic compounds in cultivated strawberries from Macedonia. Maced J Chem Chem Eng 29(2):181–194
Hager TJ, Howard LR, Liyanage R, Lay JO, Prior RL (2008) Ellagitannin composition of blackberry as determined by HPLC-ESI-MS and MALDI-TOF-MS. J Agric Food Chem 56:661–669
Okuda T, Ito H (2011) Tannins of constant structure in medicinal and food plants—hydrolysable tannins and polyphenols related to tannins. Molecules 16:2191–2217
Acknowledgements
This study was financially supported by the Statute Funds of the Institute of Food Technology and Analysis.
Author information
Authors and Affiliations
Contributions
Michał Sójka designed the research, supervised, performed the experiments and wrote the manuscript; Michał Janowski performed the experiments and analyzed the data, Katarzyna Grzelak-Błaszczyk analyzed the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sójka, M., Janowski, M. & Grzelak-Błaszczyk, K. Stability and transformations of raspberry (Rubus idaeus L.) ellagitannins in aqueous solutions. Eur Food Res Technol 245, 1113–1122 (2019). https://doi.org/10.1007/s00217-018-3212-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3212-3