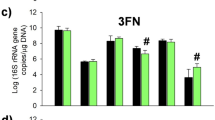
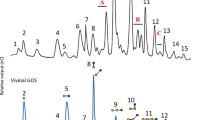
Abstract
Gut microbiota is important to human health. Specific dietary glycans promote favorable microbiota growth and inhibit pathobionts. Dietary glycans most relevant to adults and weaned infants are derived from plants or lactose; human milk oligosaccharides (HMOS) are most relevant to breastfed infants. Their efficacy in supporting bacterial growth is compared to determine their potential roles in the initiation and maintenance of colonization. Bioactivities of gluco-manno-oligosaccharides (GMOS), galacto-oligosaccharides (GOS), xylo-oligosaccharides (XOS), cellobiose (CBS), HMOS, and the most prominent individual HMOS, 2′-fucosyllactose (2′-FL) were contrasted. Two representative gut microbiota mutualists, Bifidobacteria longum ATCC15697 and Lactobacillus acidophilus NRRL B-4495, and two non-mutualists, Campylobacter jejuni S107 and Escherichia coli K12, were used to assess the in vitro prebiotic potential of these oligosaccharides. All oligosaccharides afforded growth of B. longum and L. acidophilus, with HMOS supporting the most robust growth, while none of these oligosaccharides afforded meaningful growth of non-mutualists. B. longum efficiently converted HMOS, GMOS, GOS, and XOS into organic acid fermentation products, and, to a lesser degree, L. acidophilus metabolized HMOS, GMOS, and GOS. Fermentation of these glycans by C. jejuni and E. coli was sparse. B. longum fermentation products inhibited C. jejuni and E. coli. Thus, HMOS most strongly promoted growth of the two mutualists, and both HMOS and GMOS were efficiently fermented by these mutualists into organic acids. This is consistent with a primary role of HMOS in guiding early colonization of the infant microbiota by mutualist symbionts, and of plant oligosaccharides, especially GMOS, in maintaining a favorable microbiota through adulthood.



Similar content being viewed by others
Abbreviations
- HMOS:
-
Human milk oligosaccharides
- GMOS:
-
Gluco-manno-oligosaccharides
- GOS:
-
Galacto-oligosaccharides
- XOS:
-
Xylo-oligosaccharides
- 2′-FL:
-
2′-fucosyllactose
- CBS:
-
Cellobiose
- B. longum ATCC15697:
-
Bifidobacteria longum ATCC15697
- L. acidophilus NRRL B-4495:
-
Lactobacillus acidophilus NRRL B-4495
- C. jejuni S107:
-
Campylobacter jejuni S107
- E. coli K12:
-
Escherichia coli K12
- 3-FL:
-
3-fucosyllactose
- LNT/LNnT:
-
Lacto-N-tetraose/lacto-N-neotetraose
- LNFP:
-
Lacto-N-fucopentaose
- LDFT:
-
Lactodifucotetraose
- LNDFH:
-
Lacto-N-difucohexaose
- M2:
-
Mannobiose
- M3:
-
Mannotriose
- M4:
-
Mannotetraose
- M5:
-
Mannopentaose
- M6:
-
Mannohexaose
- X2:
-
Xylobiose
- X3:
-
Xylotriose
- X4:
-
Xylotetraose
- X5:
-
Mannopentaose
- X6:
-
Xylohexaose
- LC–MS:
-
Liquid chromatography–mass spectrometry
- AA:
-
Acetic acid
- PA:
-
Propionic acid
- BA:
-
Butyric acid
- LA:
-
Lactic acid
- VA:
-
Valoric acid
- HA:
-
Hexanic acid
References
Boehm G, Jelinek J, Stahl B, Van Laere K, Knol J, Fanaro S, Moro G, Vigi V (2004) Prebiotics in infant formulas. J Clin Gastroenterol 38:76–79
Roberfroid M, Gibson GR, Hoyles L, MaCartney AL, Rastall R, Rowland I, Wolvers D, Meheust A (2010) Prebiotic effects: metabolic and health benefits. Br J Nutr 104:1–63
Ashida H, Miyake A, Kiyohara M, Wada J, Yoshida E, Kumagai H, Katayama T, Yamamoto K (2009) Two distinct α-l-fucosidases from Bifidobacterium bifidum are essential for the utilization of fucosylated milk oligosaccharides and glycoconjugates. Glycobiology 19:1010–1017
Garrido D, Ruiz-Moyano S, Jimenez-Espinoza Eom HJ, Block DE, Mills DA (2013) Utilization of galactooligosaccharides by Bifidobacterium longum subsp. infantis isolates. Food Microbiol 33:262–270
Yu Z, Chen C, Newburg DS (2013) Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 23:1281–1292
Andersen JM, Barrangou R, Hachem MA, Lahtinen S, Goh YJ, Svensson B, Klaenhammer TR (2011) Transcriptional and functional analysis of galactooligosaccharide uptake by lacS in Lactobacillus acidophilus. Proc Natl Acad Sci USA 108:17785–17790
Yu Z, Chen C, Kling DE, Liu B, McCoy JM, Merighi M, Heidtman M, Newburg DS (2013) The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology 23:169–177
Newburg DS (1996) Oligosaccharides and glycoconjugates in human milk: their role in host defense. J Mammary Gland Biol Neoplas 1:271–283
Newburg DS (2000) Oligosaccharides in human milk and bacterial colonization. J Pediatr Gastroenterol Nutr 30:8–17
Zampa A, Silvi S, Fabiani R, Morozzi G, Prpianesi C, Cresci A (2004) Effects of different digestible carbohydrates on bile acid metabolism and SCFA production by human gut micro-flora grown in an in vitro semi-continuous culture. Ecol/Environ Microbiol 10:19–26
Boehm G, Stahl B (2007) Oligosaccharides from milk. J Nutr 137:847–849
Newburg DS, Ruiz-Palacios GM, Morrow AL (2005) Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 25:37–58
He Y, Liu S, Leone S, Newburg DS (2014) Human colostrum oligosaccharides modulate major immunologic pathways of immature human intestine. Mucosal Immunol 7:1326–1339
Kunz C, Rudloff S, Baier W, Klein N, Strobe S (2000) Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr 20:699–722
González R, Klaassens ES, Malinen E, Vos WM, Vaughan EE (2008) Differential transcriptional response of Bifidobacterium longum to human milk, formula milk and galactooligasaccharide. Appl Environ Microbiol 74:4686–4694
He Y, Liu S, Kling DE, Leone S, Lawlor NT, Huang Y, Feinberg SB, Hill DR, Newburg DS (2014) The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut. doi:10.1136/gutjnl-2014-307544
Newburg DS (2013) Glycobiology of human milk. Biochemistry (Mosc) 78:771–785
Viborg AH, Katayama T, Abou Hachem M, Andersen MC, Nishimoto M, Clausen MH, Urashima T, Svensson B, Kitaoka M (2014) Distinct substrate specificities of three glycoside hydrolase family 42 β-galactosidases from Bifidobacterium longum subsp. infantis ATCC 15697. Glycobiology 24:208–216
Park A, Oh D (2010) Galacto-oligosaccharide production using microbial β-galactosidase: current state and perspectives. Appl Microbiol Biotechnol 85:1279–1286
Jones L, Seymour GB, Knox JP (1997) Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1-4)-β-D-galactan. Plant Physiol 113:1405–1412
Shabalin KA, Kulminskaya AA, Savel’ev AN, Shishlyannikov SM, Neustroev KN (2002) Enzymatic properties of α-galactosidase from Trichoderma reesei in the hydrolysis of galacto-oligosaccharides. Enzyme Microb Technol 30:231–239
Sierra C, Bernal MJ, Blasco J, Martínez R, Dalmau J, Ortuño I, Espín B, Vasallo MI, Gil D, Vidal ML, Infante D, Leis R, Maldonado J, Moreno JM, Román (2014) Prebiotic effect during the first year of life in healthy infants fed formula containing GOS as the only prebiotic: a multicenter, randomized, double-blind and placebo-controlled trial. Eur J Nutr. doi:10.1007/s00394-014-0689-9
Utami W, Meryandini A, Wiryawan KG (2013) Characterization of bacterial mannanase for hydrolyzing palm kernel cake to produce manno-oligosaccharides prebiotics. Media Peternakan 36:192–196
Gilad O, Jacobsen S, Stuer-Lauridsen B, Pedersen MB, Garrigues C, Svensson B (2010) Combined transcriptome and proteome analysis of Bifidobacterium animalis subsp. lactis BB-12 grown on xylo-oligosaccharides and a model of their utilization. Appl Environ Microbiol 76:7285–7291
Finegold SM, Li Z, Summanen PH, Downes J, Thames G, Corbett K, Dowd S, Krak M, Heber D (2014) Xylooligosaccharide increase Bifidobacteria but not Lactobacilli in human gut microbiota. Food Funct 5:436–445
Park SF, Kroll RG (1993) Expression of listeriolysin and phosphatidylinositol-specific phospholipase C is repressed by the plant-derived molecule cellobiose in Listeria monocytogenes. Mol Microbiol 8:653–661
Rastall RA, Hotchkiss AT Jr (2003) In: Eggleston G, Côté GL (eds) Oligosaccharides in food and agriculture. ACS Press, Washington, DC
Song J, Jiao LF, Xiao K, Luan ZS, Hu CH, Shi B, Zhan XA (2013) Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers. Anim Feed Sci Technol 185:175–181
Newburg DS, Pickering LK, McCluer RH, Cleary TG (1990) Fucosylated oligosaccharides of human milk protect suckling mice from heat-stabile enterotoxin of Escherichia coli. J Infect Dis 162:1075–1080
Xu Y, Fan L, Wang X, Yong Q, Yu S (2013) Simultaneous separation and quantification of linear xylo- and cello-oligosaccharides mixtures in lignocellulosics processing products on high performance anoin-exchange chromatography coupled with pulsed amperometric detection. Bioresources 8:3247–3259
Takigami S (2009) In: Phillips GO, Williams PA (eds) Handbook of hydrocolloid. Woodhead Publishing Ltd, Cambridge
Sakata S, Kitahara M, Sakamoto M, Hayashi H, Fukuyama M, Benno Y (2002) Unification of Bifidobacterium infantis and Bifidobacterium suis as Bifidobacterium longum. Int J Syst Evol Microbiol 52:1945–1951
Bao Y, Chen C, Newburg DS (2013) Quantification of neutral human milk oligosaccharides by graphitic carbon high-performance liquid chromatography with tandem mass spectrometry. Anal Biochem 433:28–35
Cimbala JM (2011) Outliers. http://www.mne.psu.edu/me345/Lectures/Outliers.pdf
Newburg DS, Ruiz-Palacios GM, Altaye M, Chaturvedi P, Meinzen-Derr J, Guerrero ML, Morrow AL (2004) Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology 14:253–263
McGrath LT, Weir CD, Maynard S, Rowlands BJ (1992) Gas-liquid chromatogrophic analysis of volatile short chain fatty acids in fecal samples as pentafluorobenzyl esters. Anal Biochem 207:227–230
Baere SD, Eeckhaut V, Steppe M, Maesschalck CD, Backer PD, Immerseel FV, Croubels SC (2013) Development of a HPLC-UV method for the quantitative determination of four short-chain fatty acids and lactic acid produced by intestinal bacteria during in vitro fermentation. J Pharm Biomed Anal 80:107–115
Garcia A, Olmo B, Lopez-Gonzalvez A, Cornejo L, Rupérez FJ, Barbas C (2008) Capillary electrophoresis for short chain organic acids in faeces reference values in a mediterranean elderly population. J Pharm Biomed Anal 46:356–361
Yang W, Adamec J, Regnier FE (2007) Enhancement of the LC/MS analysis of fatty acids through derivatization and stable isotope coding. Anal Biochem 79:5150–5157
Pettinella C, Lee SH, Cipollone F, Blair IA (2007) Targeted quantitative analysis of fatty acids in atherosclerotic plaques by high sensitivity liquid chromatography/tandem mass spectrometry. J Chromatogr B 850:168–176
Koser SA (1923) Utilization of the salts of organic acids by the colon-aerogenes group. J Bacteriol 5:493–520
Rossi M, Corradini C, Amaretti A, Nicolini M, Pompei A, Zanoni S, Matteuzzi D (2005) Fermentation of fructooligosaccharides and inulin by Bifidobacteria: a comparative study of pure and fecal cultures. Appl Environ Microbiol 71:6150–6158
Zhang M, Chen X, Zhang Z, Sun C, Chen L, He H, Zhou B, Zhang Y (2009) Purification and functional characterization of endo-β-mannanase MAN5 and its application in oligosaccharide production from konjac flour. Appl Microbiol Biotechnol 83:865–873
Wang MF, You SP, Zhang SS, Qi W, Liu ZH, Wu WN, Su RX, He ZM (2013) Purification, characterization, and production of β-mannanase from Bacillus subtilis TJ-102 and its application in gluco-mannooligosaccharides preparation. Eur Food Res Technol 237:399–408
Vernazza CL, Gibson GR, Rastall RA (2006) Carbohydrate preference, acid tolerance and bile tolerance in five strains of Bifidobacterium. J Appl Microbiol 100:846–853
Macfarlane S, Macfarlane GT, Cummings JH (2006) Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther 24:701–714
Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB (2004) Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 17:259–275
Newburg DS, Grave G (2014) Recent advances in human milk glycobiology. Pediatr Res Special article 1–5
Chapla D, Pandit P, Shah A (2012) Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresour Technol 115:215–221
Chua M, Chan K, Hocking T, Williams PA, Perry CJ, Baldwin TC (2012) Methodologies for the extraction and analysis of konjac glucomannan from corms of Amorphphallus konjac K. Koch Carbohydr Polym 87:2202–2210
Tester RS, Al-Ghazzewi FH (2013) Mannans and health, with a special focus on glucomannans. Food Res Int 50:384–391
Takahashi R, Kusakabe I, Kusama S, Sakurai Y, Murakami K, Maekawa A, Suzuki T (1984) Structures of glucomanno-oligosaccharides from the hydrolytic products of Konjac glucomannan produces by a β-mannanase from Streptomyces sp. Agric Biol Chem 48:2943–2950
Kusakabe I, Takahashi R (1988) Enzymatic preparation of β-1,4-mannooligosaccharides and β-1,4-glucomannooligosaccharides. Methods Enzymol 160:518–523
Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H (2011) Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469:543–549
Satoh T, Odamaki T, Namura M, Shimizu T, Iwatsuki K, Nishimoto M, Kitaoka M, Xiao JZ (2013) In vitro comparative evaluation of the impact of lacto-N- biose, a major buiding block of human milk oligosaccharides, on the fecal microbiota of infants. Anaerobe 19:50–57
Servin AL (2004) Antagonistic activities of Lactobacilli and Bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28:405–440
Acknowledgments
This work was supported in part by the National Institutes of Health grant number R01HD059140 (DSN), U01AI075563 (DSN) and P01HD013021 (DSN). Wang’s study was also supported by the Governmental Public Industry Research Special Funds for Projects (Grant No. 201404615), Graduate Research Innovation Projects of Jiangsu Province Ordinary University (CXZZ13_0545), and the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D.S.N. owns stock in Glycosyn, LLC, which makes human milk oligosaccharides. This potential competing financial interest is managed by Boston College. J.W., C.C., Z.Y., Y.H., and Q.Y. declare no potential conflicts of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Wang, J., Chen, C., Yu, Z. et al. Relative fermentation of oligosaccharides from human milk and plants by gut microbes. Eur Food Res Technol 243, 133–146 (2017). https://doi.org/10.1007/s00217-016-2730-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-016-2730-0