Abstract
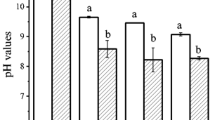
In this study, the effect of pH on the volatile generation over reaction time as well as the DPPH radical scavenging activity of volatile MRPs was investigated in the Maillard reaction of glucose (Glc) with tyrosine (Tyr) and histidine (His). Factor analysis showed clearly that volatiles generated in 5-h open system were of similar composition to those in 0.5-h close system regardless of pH level and amino acid, implying higher pressure in close system could accelerate the volatile formation in Maillard reaction. Besides, all volatiles showed increasing tendency over reaction time under different pH levels with the exception of pyrones which decreased with the extension of reaction time at pH = 7 and pH = 9. Furans, phenols and 2-acetylpyrrole that were favored under acidic condition showed larger increase at pH = 5 over reaction time, while other volatiles except pyrones, furans, phenols and 2-acetylpyrrole, which were favored under neutral and alkaline conditions, showed greater increase at pH = 7 and pH = 9 over reaction time. The measurement of DPPH radical scavenging activity of dichloromethane extract obtained from Maillard reaction mixture revealed that volatile MRPs from Glc–Tyr system showed stronger antioxidant activity under alkaline condition, while volatile MRPs from Glc–His system showed greater antioxidant activity under acidic condition.


Similar content being viewed by others
References
Buttery RG, Ling LC, Juliano BO, Turnbaugh JG (1983) Cooked rice aroma and 2-acetyl-1-pyrroline. J Agric Food Chem 31(4):823–826
Huang Y, Barringer SA (2010) Alkylpyrazines and other volatiles in cocoa liquors at pH 5 to 8, by selected ion flow tube-mass spectrometry (SIFT-MS). J Food Sci 75(1):C121–C127
Varlet V, Prost C, Serot T (2007) Volatile aldehydes in smoked fish: analysis methods, occurence and mechanisms of formation. Food Chem 105:1536–1556
Lancker FV, Adams A, Kimpe ND (2010) Formation of pyrazines in maillard model systems of lysine-containing dipeptides. J Agric Food Chem 58(4):2470–2478
Cho IH, Lee S, Jun HR, Roh HJ, Kim YS (2010) Comparison of volatile Maillard reaction products from tagatose and other reducing sugars with amino acids. Food Sci Biotechnol 19(2):431–438
Adams A, Kimpe ND (2009) Formation of pyrazines from ascorbic acid and amino acids under dry-roasting conditions. Food Chem 115(4):1417–1423
Xu H, Liu X, Zhao J, Gao Y (2008) Effects of ribose to cysteine ratios on the formation of volatile compounds from the Maillard reaction in supercritical carbon dioxide. Food Res Int 41(7):730–737
Benzing-Purdie LM, Ripmeester JA, Ratcliffe CI (1985) Effects of temperature on Maillard reaction products. J Agric Food Chem 33(1):31–33
Hill VM, Isaacs NS, Ledward DA, Ames JM (1999) Effect of high hydrostatic pressure on the volatile components of a glucose-lysine model system. J Agric Food Chem 47(9):3675–3681
Bristow M, Isaacs NS (1999) The effect of high pressure on the formation of volatile products in a model Maillard reaction. J Chem Soc Perkin Trans 2:2213–2218
Yu AN, Tan ZW, Shi BA (2011) Influence of the pH on the formation of pyrazine compounds by the Maillard reaction of l-ascorbic acid with acidic, basic and neutral amino acids. Asia-Pac J Chem Eng. doi:10.1002/apj.594
Yu AN, Zhang AD (2010) The effect of pH on the formation of aroma compounds produced by heating a model system containing l-ascorbic acid with l-threonine/l-serine. Food Chem 119(1):214–219
Yu AN, Zhang AD (2010) Aroma compounds generated from thermal reaction of l-ascorbic acid with l-cysteine. Food Chem 121(4):1060–1065
Blank I, Devaud S, Doret WM, Robert F (2003) Formation of odorants in Maillard model systems based on l-proline as affected by pH. J Agric Food Chem 51(12):3643–3650
Valero E, Sanz J, Martinez-Castro I (1999) Volatile components in microwave and conventionally-heated milk. Food Chem 66:333–338
Ji H, Bernhard RA (1992) Effect of microwave heating on pyrazine formation in a model system. J Sci Food Agric 59:283–289
Hodge JE (1953) Chemistry of browning reactions in model systems. J Agric Food Chem 1(15):928–943
Illmann S, Davidek T, Gouézec E, Rytz A, Schuchmann HP, Blank I (2009) Generation of 4-hydroxy-2, 5-dimethyl-3(2H)-furanone from rhamnose as affected by reaction parameters: experimental design approach. J Agric Food Chem 57(7):2889–2895
Ames JM, Guy RCE, Kipping GJ (2001) Effect of pH and temperature on the formation of volatile compounds in cysteine/reducing sugar/starch mixtures during extrusion cooking. J Agric Food Chem 49:1885–1894
Cerny C, Briffod M (2007) Effect of pH on the Maillard reaction of [13C5]xylose, cysteine, and thiamin. J Agric Food Chem 55(4):1552–1556
Ames JM, Guy RCE, Kipping GJ (2001) Effect of pH, temperature, and moisture on the formation of volatile compounds in glycine/glucose model systems. J Agric Food Chem 49(9):4315–4323
Wei GJ, Ho CT, Huang AS (2009) Determination of volatile compounds formed in a glucose-selenomethionine model system by gas chromatography-atomic emission detector and gas chromatography-mass spectrometry. Food Chem 116(3):774–778
Rizzi GP (2004) Role of phosphate and carboxylate ions in Maillard browning. J Agric Food Chem 52(4):953–957
Osorio C, Alarcon M, Moreno C, Bonilla A, Barrios J, Garzon C, Duque C (2006) Characterization of odar-active volatiles in champa (Campomanesia lineatifolia R. & P.). J Agric Food Chem 54(2):509–516
Deport C, Ratel J, Berdagué JL, Engel E (2006) Comprehensive combinatory standard correcction: A calibration method for handling instrumental drifts of gas chromatography-mass spectrometry systems. J Chromatogr A 1116(1–2):248–258
Beal AD, Mottram DS (1994) Compounds contributing to the characteristic aroma of malted barley. J Agric Food Chem 42(12):2880–2884
Mihara S, Nishimura O (1989) Retention indices of 2-hydroxy-2-cyclopenten-1-ones. J High Resolut Chromatogr 12:763–764
Güntert M, Rapp A, Takeoka GR, Jennings W (1986) HRGC and HRGC-MS applied to wine constituents of lower volatility. Z Lebensm Unters Forsch 182(3):200–204
Shi WH, Sun WW, Yu SJ, Zhao MM (2010) Study on the characteristic of bovine serum albumin-glucose model system, treated by ultrasonic. Food Res Int 43:2115–2118
Weenen H (1998) Reactive intermediates and carbohydrate fragmentation in Maillard chemistry. Food Chem 62(4):393–401
Nursten H (2005) The Maillard reaction: chemistry, biochemistry and implications. Royal society of chemistry, Cambridge
Nishimura O, Mihara S (1990) Investigation of 2-hydroxy-2-cyclopenten-1-ones in roasted coffee. J Agric Food Chem 38(4):1038–1041
Niemela K (1988) The formation of 2-hydroxy-2-cyclopenten-1-ones from polysaccarides during kraft pulping of pine wood. Carbohydr Res 184:131–137
Adams A, Polizzi V, Boekel MV, Norbert DK (2008) Formation of pyrazines and a novel pyrrole in Maillard model systems of 1, 3-dihydroxyacetone and 2-oxopropanal. J Agric Food Chem 56(6):2147–2153
Cechovská L, Cejpek K, Konecny M, Velisek J (2011) On the role of 2,3-dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one in antioxidant capacity of prunes. E Food Res Technol 233:367–378. doi:10.1007/s00217-011-1527-4
Wang Y, Juliani HR, Simon JE, Ho CT (2009) Amino acid-dependent formation pathways of 2-acetylfuran and 2, 5-dimethyl-4-hydroxy-3[2H]-furanone in the Maillard reaction. Food Chem 115(1):233–237
Gi U-S, Baltes W (1995) Model reactions on roast aroma formation. 15. Investigations on the formation of pyrido[3, 4-d]imidazoles during the Maillard reaction. J Agric Food Chem 43(8):2226–2230
Eiserich JP, Shibamoto T (1994) Antioxidative activity of volatile heterocyclic compounds. J Agric Food Chem 42(5):1060–1063
Eiserich JP, Macku C, Shibamoto T (1992) Volatile antioxidants formed from an l-cysteine/d-glucose Maillard model system. J Agric Food Chem 40(10):1982–1988
Yanagimoto K, Lee KG, Ochi H, Shibamoto T (2002) Antioxidant activity of heterocyclic compounds found in coffee volatiles produced by Maillard reaction. J Agric Food Chem 50(19):5480–5484
Acknowledgments
This research is supported by a grant from Zhengzhou Tobacco Research Institute of CNTC (422010CZ0600).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, X., Zhao, M., Hu, J. et al. Influence of pH on the formation and radical scavenging activity of volatile compounds produced by heating glucose with histidine/tyrosine. Eur Food Res Technol 234, 333–343 (2012). https://doi.org/10.1007/s00217-011-1644-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-011-1644-0