Abstract
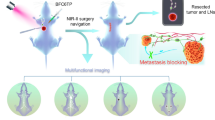
A viscosity-sensitive, lysosome-targeted near-infrared fluorescent probe (PYATT) was reported in this paper. The fluorescent spectra of PYATT are strongly dependent on viscosity, resulting in a Stokes shift of about 190 nm. Given its photostability, low cytotoxicity, and high fluorescence quantum yield, PYATT is expected to be used in cell imaging. Due to the higher viscosity of tumor cells than normal cells, the fluorescence intensity of PYATT in tumor cells is higher than normal cells, which can realize the visualization of tumors. The near-infrared probe (PYATT) is viscosity-dependent in lysosomes, which is valuable in early diagnosis and treatment of tumor.
Graphical Abstract







Similar content being viewed by others
References
Ludwanowski S, Samanta A, Loescher S, Barner-Kowollik C, Walther A. A modular fluorescent probe for viscosity and polarity sensing in DNA hybrid mesostructures. Adv Sci. 2021;8(5):2003740. https://doi.org/10.1002/advs.202003740.
Robson JA, Kubánková M, Bond T, Hendley RA, White AJP, Kuimova MK, Wilton-Ely JDET. Simultaneous detection of carbon monoxide and viscosity changes in cells. Angew Chem Int Ed. 2020;59(48):21431–5. https://doi.org/10.1002/anie.202008224.
Wang YN, Zhao XQ, Qiu LH, Sun R, Xu YJ, Ge JF. Viscosity sensitive endoplasmic reticulum fluorescent probes based on oxazolopyrdinium. J Mater Chem B. 2021;9(28):5664–9. https://doi.org/10.1039/D1TB01106E.
Feng S, Gong S, Zheng Z, Feng G. Smart dual-response probe reveals an increase of GSH level and viscosity in cisplatin-induced apoptosis and provides dual-channel imaging for tumor. Sensor Actuat B-Chem. 2021;351: 130940. https://doi.org/10.1016/j.snb.2021.130940.
Li S, Wang P, Feng W, Xiang Y, Dou K, Liu Z. Simultaneous imaging of mitochondrial viscosity and hydrogen peroxide in Alzheimer’s disease by a single near-infrared fluorescent probe with a large Stokes shift. Chem Commun. 2019;56(7):1050–3. https://doi.org/10.1039/c9cc08267k.
Shen B, Wang L, Zhi X, Qian Y. Construction of a red emission BODIPY-based probe for tracing lysosomal viscosity changes in culture cells. Sensor Actuat B-Chem. 2019;304: 127271. https://doi.org/10.1016/j.snb.2019.127271.
Kim Y, Choi M, Mulay SV, Jang M, Kim JY, Lee WH, Jon S, Churchill DG. Aqueous red-emissive probe for the selective fluorescent detection of cysteine by deprotection/cyclization cascade resulting in large stokes’ shift. Chem Eur J. 2018;24(21):5623–9. https://doi.org/10.1002/chem.201706073.
Gao Y, Hu Y, Liu Q, Li XK, Li XM, Kim CY, James TD, Li J, Chen X, Guo Y. Two-dimensional design strategy to construct smart fluorescent probes for the precise tracking of senescence. Angew Chem Int Ed. 2021;60(19):10756–65. https://doi.org/10.1002/anie.202101278.
Piazzolla F, Mercier V, Assies L, Sakai N, Roux A, Matile S. Fluorescent membrane tension probes for early endosomes. Angew Chem Int Ed. 2021;60(22):12258–63. https://doi.org/10.1002/anie.202016105.
Reja SI, Minoshima M, Hori Y, Kikuchi K. Near-infrared fluorescent probes: a next-generation tool for protein-labeling applications. Chem Sci. 2021;12(10):3437–47. https://doi.org/10.1039/d0sc04792a.
Georgiev NI, Marinova NV, Bojinov VB. Design and synthesis of light-harvesting rotor based on 1,8-naphthalimide units. J Photoch Photobio A. 2020;401: 112733. https://doi.org/10.1016/j.jphotochem.2020.112733.
Yan F, Sun X, Ma T, Zhang Y, Jiang Y, Wang R, Ma C, Wei J, Chen L, Cui Y. A viscosity-dependent carbon dots with anti-VEGF properties for monitoring and promoting apoptosis in cancerous cell. Chem Eng J. 2021;407: 127801. https://doi.org/10.1016/j.cej.2020.127801.
Zhou Y, Liu Z, Qiao G, Tang B, Li P. Visualization of endoplasmic reticulum viscosity in the liver of mice with nonalcoholic fatty liver disease by a near-infrared fluorescence probe. Chinese Chem Lett. 2021;32(11):3641–5. https://doi.org/10.1016/j.cclet.2021.04.035.
Boyle B, Dive C, Fitzgerald R, Hanna GB, Hill S, Hunter D, Janes S, Kaye S, Kumar H, Oien K, Palmer C, Richards A, Richards M, Sasieni P, Steele B, Walter F. A roadmap for the early detection and diagnosis of cancer. Lancet Oncol. 2020;21(11):1397–9. https://doi.org/10.1016/S1470-2045(20)30593-3.
Chinen AB, Guan CM, Ferrer JR, Barnaby SN, Merkel TJ, Mirkin CA. Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chem Rev. 2015;115(19):10530–74. https://doi.org/10.1021/acs.chemrev.5b00321.
Li ZP, Wang YF, Zeng CC, Hu LM, Liang XJ. Ultrasensitive tyrosinase activated turn-on near-infrared fluorescent probe with a rationally designed urea bond for selective imaging and photodamage to melanoma cells. Anal Chem. 2018;90(6):3666–9. https://doi.org/10.1021/acs.analchem.7b05369.
Liu JN, Bu WB, Shi JL. Chemical design and synthesis of functionalized probes for imaging and treating tumor hypoxia. Chem Rev. 2017;117(9):6160–224. https://doi.org/10.1021/acs.chemrev.6b00525.
Jiang L, Chen T, Song E, Fan Y, Min D, Zeng L, Bao GM. High-performance near-infrared fluorescence probe for fast and specific visualization of harmful sulfite in food, living cells, and zebrafish. Chem Eng J. 2022;427: 131563. https://doi.org/10.1016/j.cej.2021.131563.
Zong C, Lu Q, Niu J, Meng F, Yu X. A fluorescent probe for detecting mitochondrial viscosity and its application in distinguishing human breast cancer cells from normal ones. Spectrochim Acta A. 2023;299: 122883. https://doi.org/10.1016/j.saa.2023.122883.
Yang L, Gu P, Fu A, Xi Y, Cui S, Ji L, Li L, Ma N, Wang Q, He G. TPE-based fluorescent probe for dual channel imaging of pH/viscosity and selective visualization of cancer cells and tissues. Talanta. 2023;265: 124862. https://doi.org/10.1016/j.talanta.2023.124862.
Han S, Yang L, Liu M, Li H, Song X. Accurate diagnosis of hepatic fibrosis with dual detection of nitric oxide and viscosity by a ratiometric fluorescent probe. Chem Eng J. 2023;463: 142383. https://doi.org/10.1016/j.cej.2023.142383.
Zheng F, Ding J, Huang S, Bi A, Liu S, Zhang K, Chen F, Zeng W. Real-time monitoring viscosity during ferroptosis with a novel mitochondria-specific fluorescent probe. Dyes Pigments. 2023;217: 111424. https://doi.org/10.1016/j.dyepig.2023.111424.
Dou K, Huang W, Xiang Y, Li S, Liu Z. Design of activatable NIR-II molecular probe for in vivo elucidation of disease-related viscosity variations. Anal Chem. 2020;92(6):4177–81. https://doi.org/10.1021/acs.analchem.0c00634.
Shi WJ, Yang J, Wei YF, Li XT, Yan XH, Wang Y, Leng H, Zheng L, Yan JW. Novel cationic meso-CF3 BODIPY-based AIE fluorescent rotors for imaging viscosity in mitochondria. Chem Commun. 2022;58(12):1930–3. https://doi.org/10.1039/d1cc06532g.
Liu Y, Feng S, Gong S, Feng G. Dual-channel fluorescent probe for detecting viscosity and ONOO− without signal crosstalk in nonalcoholic fatty liver. Anal Chem. 2022;94(50):17439–47. https://doi.org/10.1021/acs.analchem.2c03419.
Kim SJ, Park SY, Yoon SA, Kim C, Kang C, Lee MH. Naphthalimide-4-(4-nitrophenyl)thiosemicarbazide: a fluorescent probe for simultaneous monitoring of viscosity and nitric oxide in living cells. Anal Chem. 2021;93(10):4391–7. https://doi.org/10.1021/acs.analchem.0c04019.
Wang L, Xiao Y, Tian W, Deng L. Activatable rotor for quantifying lysosomal viscosity in living cells. J Am Chem Soc. 2013;135(8):2903–6. https://doi.org/10.1021/ja311688g.
Zhang Z, Kang M, Tan H, Song N, Li M, Xiao P, Yan D, Zhang L, Wang D, Tang BZ. The fast-growing field of photo-driven theranostics based on aggregation-induced emission. Chem Soc Rev. 2022;51(6):1983–2030. https://doi.org/10.1039/d1cs01138c.
Würthner F. Aggregation-induced emission (AIE): a historical perspective. Angew Chem Int Ed. 2020;59(34):14192–6. https://doi.org/10.1002/anie.202007525.
Feng S, Liu Y, Li Q, Gui Z, Feng G. Two water-soluble and wash-free fluorogenic probes for specific lighting up cancer cell membranes and tumors. Anal Chem. 2022;94(3):1601–7. https://doi.org/10.1021/acs.analchem.1c03685.
Fu L, Zhao W, Tan Y, Ding Y, Wang Y, Qing W. Rational design of water-soluble mitochondrial-targeting near-infrared fluorescent probes with large Stokes shift for distinguishing cancerous cells and bioimaging. Spectrochim Acta A. 2023;299: 122869. https://doi.org/10.1016/j.saa.2023.122869.
Fu L, Tan Y, Ding Y, Qing W, Wang Y. Water-soluble and polarity-sensitive near-infrared fluorescent probe for long-time specific cancer cell membranes imaging and C. elegans label. Chinese Chem Lett. 2023. https://doi.org/10.1016/j.cclet.2023.108886.
Leung MCK, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci. 2008;106(1):5–28. https://doi.org/10.1093/toxsci/kfn121.
Wang H, Sun Y, Lin X, Feng W, Li Z, Yu M. Multi-organelle-targeting pH-dependent NIR fluorescent probe for lysosomal viscosity. Chinese Chem Lett. 2023;34(3): 107626. https://doi.org/10.1016/j.cclet.2022.06.049.
Wu X, Wang X, Li Y, Kong F, Xu K, Li L, Tang B. A near-infrared probe for specific imaging of lipid droplets in living cells. Anal Chem. 2022;94(11):4881–8. https://doi.org/10.1021/acs.analchem.2c00651.
Wang X, Chen Q, Dong K, Sun C, Huang Y, Qiang Z, Chen B, Chen M, Feng Y, Meng X. Accurate monitoring and multiple evaluations of mitophagy by a versatile two-photon fluorescent probe. Anal Chem. 2021;93(26):9200–8. https://doi.org/10.1021/acs.analchem.1c01365.
Funding
This work was financially supported by the Henan Provincial Science and Technology Research Project of China (No. 232102310369).
Author information
Authors and Affiliations
Contributions
Y.W.: supervision, conceptualization, and project administration. L.F.: methodology and formal analysis. Y.T.: formal analysis and validation. Y.D.: software. W.Q.: writing, review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Fu, L., Tan, Y. et al. A near-infrared fluorescent probe with viscosity sensitivity in lysosome for cancer visualization. Anal Bioanal Chem 416, 341–348 (2024). https://doi.org/10.1007/s00216-023-05050-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-05050-6