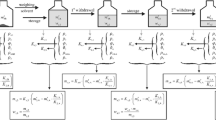
Abstract
The National Institute of Standards and Technology, which is the national metrology institute of the USA, assigns certified values to the mass fractions of individual elements in single-element solutions, and to the mass fractions of anions in anion solutions, based on gravimetric preparations and instrumental methods of analysis. The instrumental method currently is high-performance inductively coupled plasma optical emission spectroscopy for the single-element solutions, and ion chromatography for the anion solutions. The uncertainty associated with each certified value comprises method-specific components, a component reflecting potential long-term instability that may affect the certified mass fraction during the useful lifetime of the solutions, and a component from between-method differences. Lately, the latter has been evaluated based only on the measurement results for the reference material being certified. The new procedure described in this contribution blends historical information about between-method differences for similar solutions produced previously, with the between-method difference observed when a new material is characterized. This blending procedure is justified because, with only rare exceptions, the same preparation and measurement methods have been used historically: in the course of almost 40 years for the preparation methods, and of 20 years for the instrumental methods. Also, the certified values of mass fraction, and the associated uncertainties, have been very similar, and the chemistry of the solutions also is closely comparable within each series of materials. If the new procedure will be applied to future SRM lots of single-element or anion solutions routinely, then it is expected that it will yield relative expanded uncertainties that are about 20 % smaller than the procedure for uncertainty evaluation currently in use, and that it will do so for the large majority of the solutions. However, more consequential than any reduction in uncertainty, is the improvement in the quality of the uncertainty evaluations that derives from incorporating the rich historical information about between-method differences and about the stability of the solutions over their expected lifetimes. The particular values listed for several existing SRMs are given merely as retrospective illustrations of the application of the new method, not to suggest that the certified values or their associated uncertainties should be revised.
Graphical abstract





Similar content being viewed by others
Notes
The IUPAC Orange Book [7, 10.3.4] recommends that the term OES be abandoned and that AES (denoting atomic emission spectroscopy/spectrometry) be used instead, while noting that both OES and AES have been advocated in IUPAC documents. This contribution concerns historical information from the analytical methods we have been using for the certification of the solutions being discussed. Considering that users of these solutions are most familiar with the usage of OES in many relevant publications, including those just cited, to avoid confusion we will continue to refer to AES methods using the traditional designation “OES.”
References
Beauchamp CR, Camara JE, Carney J, Choquette SJ, Cole KD, DeRose PC, Duewer DL, Epstein MS, Kline MC, Lippa KA, Lucon E, Phinney KW, Possolo A, Sharpless KE, Sieber JR, Toman B, Winchester MR, Windover D. Metrological tools for the reference materials and reference instruments of the NIST Materials Measurement Laboratory. In: NIST Special Publication 260-136 (2020 Edition). National Institute of Standards and Technology. 2020. https://doi.org/10.6028/NIST.SP.260-136-2020
Salit ML, Turk GC. A drift correction procedure. Analytical Chemistry. 1998;70(15):3184–90. https://doi.org/10.1021/ac980095b.
Salit ML, Turk GC, Lindstrom AP, Butler TA, Beck CM, Norman B. Single-element solution comparisons with a high-performance inductively coupled plasma optical emission spectrometric method. Analytical Chemistry. 2001;73(20):4821–9. https://doi.org/10.1021/ac0155097.
Salit ML, Turk GC. Traceability of single-element calibration solutions. Analytical Chemistry. 2005;77(7):136–41. https://doi.org/10.1021/ac053354n.
Winchester MR, Butler TA, Turk GC. Improving the high-performance inductively coupled plasma optical emission spectrometry methodology through exact matching. Analytical Chemistry. 2010;82(18):7675–83. https://doi.org/10.1021/ac101471a.
Brennan RG, Butler TA, Winchester MR. Achieving 0.2 % relative expanded uncertainty in ion chromatography analysis using a high-performance methodology. Analytical Chemistry. 2011;83(10):3801–7. https://pubs.acs.org/doi/10.1021/ac200290y
Inczedy J, Lengyel T, Ure AM, of Pure IU, Chemistry A. IUPAC Compendium on Analytical Nomenclature, Definitive Rules 1997, 3rd edn. Blackwell Science. 1998. IUPAC Orange Book. https://media.iupac.org/publications/analytical_compendium/
Joint Committee for Guides in Metrology (JCGM): Evaluation of Measurement Data — Guide to the Expression of Uncertainty in Measurement. International Bureau of Weights and Measures (BIPM), Sèvres, France. 2008. BIPM, IEC, IFCC, ILAC, ISO, IUPAC, IUPAP and OIML, JCGM 100:2008, GUM 1995 with minor corrections. https://www.bipm.org/en/publications/guides/gum.html
Koepke A, Lafarge T, Toman B, Possolo A. NIST Consensus Builder — User’s Manual. National Institute of Standards and Technology. 2017. National Institute of Standards and Technology. https://consensus.nist.gov
DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–88. https://doi.org/10.1016/0197-2456(86)90046-2.
Koepke A, Lafarge T, Possolo A, Toman B. Consensus building for interlaboratory studies, key comparisons, and meta-analysis. Metrologia. 2017;54(3):34–62. https://doi.org/10.1088/1681-7575/aa6c0e.
Thompson M, Ellison SLR. Dark uncertainty. Accreditation and Quality Assurance. 2011;16:483–7. https://doi.org/10.1007/s00769-011-0803-0.
Hoaglin DC. Misunderstandings about \(Q\) and ‘Cochran’s \(Q\) test’ in meta-analysis. Statistics in Medicine. 2016;35:485–95. https://doi.org/10.1002/sim.6632.
Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, Kuss O, Higgins JPT, Langan D, Salanti G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Research Synthesis Methods. 2016;7:55–79. https://doi.org/10.1002/jrsm.1164.
Langan D, Higgins JPT, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, Viechtbauer W, Simmonds M. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Research Synthesis Methods. 2019;10(1):83–98. https://doi.org/10.1002/jrsm.1316.
Weber F, Knapp G, Glass A, Kundt G, Ickstadt K. Interval estimation of the overall treatment effect in random-effects meta-analyses: Recommendations from a simulation study comparing frequentist, Bayesian, and bootstrap methods. Research Synthesis Methods. 2021;12(3):291–315. https://doi.org/10.1002/jrsm.1471.
Strawderman WE, Rukhin AL. Simultaneous estimation and reduction of nonconformity in interlaboratory studies. Journal of the Royal Statistical Society Series B (Statistical Methodology). 2010;72(2):219–34. https://doi.org/10.2307/40541584.
Possolo A, Bodnar O, Butler TA, Molloy JL, Winchester MR. Value assignment and uncertainty evaluation in single-element reference solutions. Metrologia. 2018;55(3):404–13. https://doi.org/10.1088/1681-7575/aabd57.
Possolo A, Toman B. Tutorial for metrologists on the probabilistic and statistical apparatus underlying the gum and related documents. National Institute of Standards and Technology, 2011. https://doi.org/10.13140/RG.2.1.2256.8482. https://www.itl.nist.gov/div898/possolo/TutorialWEBServer/TutorialMetrologists2011Nov09.xht
Kipphardt H, Matschat R, Rienitz O, Schiel D, Gernand W, Oeter D. Traceability system for elemental analysis. Accreditation and Quality Assurance. 2006;10(11):633–9. https://doi.org/10.1007/s00769-005-0084-6.
Westwood S, Choteau T, Daireaux A, Josephs RD, Wielgosz RI. Mass balance method for the SI value assignment of the purity of organic compounds. Analytical Chemistry. 2013;85(6):3118–26. https://doi.org/10.1021/ac303329k.
Vogl J, Kipphardt H, Richter S, Bremser W, Torres MRA, Manzano JVL, Buzoianu M, Hill S, Petrov P, Goenaga-Infante H, Sargent M, Fisicaro P, Labarraque G, Zhou T, Turk GC, Winchester M, Miura T, Methven B, Sturgeon R, Jährling R, Rienitz O, Mariassy M, Hankova Z, Sobina E, Krylov AI, Kustikov YA, Smirnov VV. Establishing comparability and compatibility in the purity assessment of high purity zinc as demonstrated by the CCQM-p149 intercomparison. Metrologia. 2018;55(2):211–21. https://doi.org/10.1088/1681-7575/aaa677.
Linsinger TPJ, Pauwels J, Lamberty A, Schimmel HG, van der Veen AMH, Siekmann L. Estimating the uncertainty of stability for matrix CRMs. Fresenius’ Journal of Analytical Chemistry. 2001;370:183–8. https://doi.org/10.1007/s0021601007.
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29. https://doi.org/10.2307/3001666.
Welch BL. The generalization of ‘Student’s’ problem when several different population variances are involved. Biometrika. 1947;34:28–35. https://doi.org/10.1093/biomet/34.1-2.28.
Analytical Methods Committee. Dark uncertainty. Analytical Methods. 2012;4:2609–12. https://doi.org/10.1039/C2AY90034C. AMC Technical Briefs No. 53.
Searle SR, Casella G, McCulloch CE. Variance components. John Wiley & Sons, 2006
Horwitz W, Albert R. The Horwitz Ratio (HorRat): A useful index of method performance with respect to precision. Journal of AOAC International. 2006;89(4):1095–109. https://doi.org/10.1093/jaoac/89.4.1095.
Meija J. A chemical uncertainty principle challenge. Analytical and Bioanalytical Chemistry. 2007;387:1583–4. https://doi.org/10.1007/s00216-006-1059-0.
Meija J. Solution to the chemical uncertainty principle challenge. Analytical and Bioanalytical Chemistry. 2007;388:995–6. https://doi.org/10.1007/s00216-007-1312-1.
Sieber JR, Epstein MS, Possolo AM. A Retuned Horwitz procedure for upgrading certificates of older standard reference materials. NIST Special Publication 260-198. National Institute of Standards and Technology, Gaithersburg, MD, 2019. https://doi.org/10.6028/NIST.SP.260-198
Horwitz W. Evaluation of analytical methods used for regulation of foods and drugs. Analytical Chemistry. 1982;54(1):67–76. https://doi.org/10.1021/ac00238a765.
McCulloch CE, Searle SR, Neuhaus JM. Generalized, Linear, and Mixed Models, 2nd edn. John Wiley & Sons, 2008
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evidence-Based Mental Health. 2019;22:153–60. https://doi.org/10.1136/ebmental-2019-300117.
R Core Team: R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2021. R Foundation for Statistical Computing. https://www.R-project.org/
Langan D, Higgins JPT, Simmonds M. Comparative performance of heterogeneity variance estimators in meta-analysis: a review of simulation studies. Research Synthesis Methods. 2017;8(2):181–98. https://doi.org/10.1002/jrsm.1198.
Maechler M, Rousseeuw P, Croux C, Todorov V, Ruckstuhl A, Salibian-Barrera M, Verbeke T, Koller M, Conceição ELT, di Palma MA. Robustbase: Basic Robust Statistics. 2021. R package version 0.93-9. http://CRAN.R-project.org/package=robustbase
Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge University Press. 2007 https://doi.org/10.1017/CBO9780511790942.
Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian data analysis, 2nd edn. Chapman & Hall / CRC, 2003
Carpenter B, Gelman A, Hoffman M, Lee D, Goodrich B, Betancourt M, Brubaker M, Guo J, Li P, Riddell A. Stan: A probabilistic programming language. Journal of Statistical Software. 2017;76(1):1–32. https://doi.org/10.18637/jss.v076.i01.
Freedman D, Pisani R, Purves R. Statistics, 4th edn. W. W. Norton & Company, 2007
Yu LL, Butler TA, Turk GC. Effect of valence state on ICP-OES value assignment of SRM 3103a arsenic spectrometric solution. Analytical Chemistry. 2006;78:1651–6. https://doi.org/10.1021/ac051732i.
Narukawa T, Kuroiwa T, Chiba K. Mechanism of sensitivity difference between trivalent inorganic As species [As(III)] and pentavalent species [As(V)] with inductively coupled plasma spectrometry. Talanta. 2007;73:157–65. https://doi.org/10.1016/j.talanta.2007.03.021.
Narukawa T, Chiba K, Kuroiwa T, Inagaki K. Differences in sensitivity between As(III) and As(V) measured by inductively coupled plasma spectrometry and the factors affecting the incoherent molecular formation (IMF) effect in the plasma. Journal of Analytical Atomic Spectrometry. 2010;25:1682–7. https://doi.org/10.1039/C0JA00011F.
Levenson MS, Banks DL, Eberhardt KR, Gill LM, Guthrie WF, Liu HK, Vangel MG, Yen JH, Zhang NF. An approach to combining results from multiple methods motivated by the ISO GUM. Journal of Research of the National Institute of Standards and Technology. 2000;105(4):571–9. https://doi.org/10.6028/jres.105.047.
Vangel MG, Rukhin AL. Maximum likelihood analysis for heteroscedastic one-way random effects ANOVA in interlaboratory studies. Biometrics. 1999;55:129–36. https://doi.org/10.1111/j.0006-341X.1999.00129.x.
Rukhin A, Biggerstaff B, Vangel M. Restricted maximum likelihood estimation of a common mean and the Mandel-Paule algorithm. Journal of Statistical Planning and Inference. 2000;83:319–30. https://doi.org/10.1016/S0378-3758(99)00098-1.
Toman B. Bayesian approaches to calculating a reference value in key comparison experiments. Technometrics. 2007;49(1):81–7. https://doi.org/10.1198/004017006000000273.
Moody JR, Greenberg RR, Pratt KW, Rains TC. Recommended inorganic chemicals for calibration. Analytical Chemistry. 1988;60(21):1203–18. https://doi.org/10.1021/ac00172a001.
Acknowledgements
The authors are grateful to their NIST colleagues who kindly provided detailed reviews of a draft, and offered useful comments and suggestions for improvement: Michael Epstein, Adam Pintar, and Michael Winchester. The authors are also highly appreciative of the suggestions for improvement offered by two anonymous reviewers, in particular for pointing out the similarity between Eq. (2) and the Horwitz equation [28].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no competing interests. The research reported herein did not involve human or animal subjects, or any biological materials, as objects of research. This research was conducted as part of the authors’ duties as employees of the National Institute of Standards and Technology, an agency of the federal government of the United States of America, under the U.S. Department of Commerce.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lang, B.E., Molloy, J.L., Vetter, T.W. et al. Value assignment and uncertainty evaluation for anion and single-element reference solutions incorporating historical information. Anal Bioanal Chem 415, 1657–1673 (2023). https://doi.org/10.1007/s00216-022-04410-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04410-y