Abstract
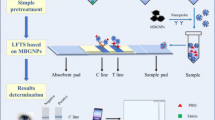
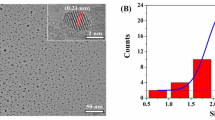
Dicamba herbicide is increasingly used in the world, in particular‚ with the widespread cultivation of genetically modified dicamba-resistant crops. However, the drift problem in the field has caused phytotoxicity against naive, sensitive crops, raising legal concerns. Thus, it is particularly timely to develop a method that can be used for on-the-spot rapid detection of dicamba in the field. In this paper, a lateral flow immunochromatographic strip (LFIC) was developed. The quantitative detection can be conducted by an app on a smartphone, named “Color Snap.” The tool reported here provides results in 10 min and can detect dicamba in water with a LOD (detection limit) value of 0.1 mg/L. The developed LFIC shows excellent stability and sensitivity appropriate for field analysis. Our sensor is portable and excellent tool for on-site detection with smartphone imaging for better accuracy and precision of the results.







Similar content being viewed by others
References
Jones GT, Norsworthy JK, Barber T. Off-target movement of diglycolamine dicamba to non-dicamba soybean using practices to minimize primary drift. Weed Technol. 2019;33(1):24–40.
Shaner D L. Herbicide handbook. Weed Science Society of America. Lawrence; 2014.
Werle R, Oliveira MC, Jhala AJ, Proctor CA, Rees J, Klein R. Survey of Nebraska farmers’ adoption of dicamba-resistant soybean technology and dicamba off-target movement. Weed Technol. 2018;32(6):754–61.
Lee TC, Edrington TC, Bell E, Burzio LA, Glenn KC. Effect of common processing of soybeans on the enzymatic activity and detectability of the protein, dicamba mono-oxygenase (DMO), introduced into dicamba-tolerant MON 87708. Regul Toxicol Pharmacol. 2019;102:98–107.
James C. Global status of commercialized biotech/GM crops in 2017: Biotech crop adoption surges as economic benefits accumulate in 22 years. ISAAA Brief No. 53.: The International Service for the Acquisition of Agri-biotech Applications (ISAAA); 2017.
Sciumbato AS, Chandler JM, Senseman SA, Bovey RW, Smith KL. Determining exposure to auxin-like herbicides. I. Quantifying injury to cotton and soybean. Weed Technol. 2004;18(4):1125–34.
Behrens R, Lueschen W. Dicamba volatility. Weed Sci. 1979;27(5):486–93.
Grover R, Maybank J, Yoshida K. Droplet and vapor drift from butyl ester and dimethylamine salt of 2, 4-D. Weed Sci. 1972;20(4):320–4.
Jones GT, Norsworthy JK, Barber T, Gbur E, Kruger GR. Off-target movement of DGA and BAPMA dicamba to sensitive soybean. Weed Technol. 2019;33(1):51–65.
Bruns V. The response of certain crops to 2, 4-dichlorophenoxy-acetic acid in irrigation water: part I. Red Mexican Beans Weeds. 1954;3(4):359–76.
Culpepper AS, Sosnoskie LM, Shugart J, Leifheit N, Curry M, Gray T. Effects of low-dose applications of 2, 4-D and dicamba on watermelon. Weed Technol. 2018;32(3):267–72.
Egan JF, Bohnenblust E, Goslee S, Mortensen D, Tooker J. Herbicide drift can affect plant and arthropod communities. Agric Ecosyst Environ. 2014;185:77–87.
Hill B, Harker K, Hasselback P, Moyer J, Inaba D, Byers S. Phenoxy herbicides in Alberta rainfall: potential effects on sensitive crops. Can J Plant Sci. 2002;82(2):481–4.
Johnson VA, Fisher LR, Jordan DL, Edmisten KE, Stewart AM, York AC. Cotton, peanut, and soybean response to sublethal rates of dicamba, glufosinate, and 2, 4-D. Weed Technol. 2012;26(2):195–206.
Caux P-Y, Kent R, Tache M, Grande C, Fan G, MacDonald D. Environmental fate and effects of dicamba: a Canadian perspective. In: Reviews of environmental contamination and toxicology. New York Springer 1980. pp. 1–58.
Perocco P, Ancora G, Rani P, Valenti A, Mazzullo M, Colacci A, Grilli S. Evaluation of genotoxic effects of the herbicide dicamba using in vivo and in vitro test systems. Environ Mol Mutagen. 1990;15(3):131–5.
Reuter W (2019) Toxicology of glyphosate, isoxaflutole, dicamba and possible combination effects. Testbiotech https://doi.org/www.testbiotech.org/sites/default/files/Tox_Evaluation_Glyphosate_Dicam ba_Isoxaflutole.pdf Accessed 18.
González N, Soloneski S, Larramendy M. Genotoxicity analysis of the phenoxy herbicide dicamba in mammalian cells in vitro. Toxicol in Vitro. 2006;20(8):1481–7.
Xiong W, Tao X, Pang S, Yang X, Tang G, Bian Z. Separation and quantitation of three acidic herbicide residues in tobacco and soil by dispersive solid-phase extraction and UPLC–MS/MS. J Chromatogr Sci. 2014;52(10):1326–31.
Sturm J, Wienhold P, Frenzel T, Speer K. Ultra Turrax® tube drive for the extraction of pesticides from egg and milk samples. Anal Bioanal Chem. 2018;410(22):5431–8.
Guo H, Riter LS, Wujcik CE, Armstrong DW. Quantitative analysis of dicamba residues in raw agricultural commodities with the use of ion-pairing reagents in LC–ESI–MS/MS. Talanta. 2016;149:103–9.
Clegg BS, Stephenson GR, Hall JC. Development of an enzyme-linked immunosorbent assay for the detection of dicamba. J Agric Food Chem. 2001;49(5):2168–74.
Huo JQ, Barnych B, Li ZF, Wan DB, Li DY, Vasylieva N, Knezevic SZ, Osipitan OA, Scott JE, Zhang JL, Hammock BD. Hapten synthesis, antibody development, and a highly sensitive indirect competitive chemiluminescent enzyme immunoassay for detection of dicamba. J Agric Food Chem. 2019;67(20):5711–9. https://doi.org/10.1021/acs.jafc.8b07134.
Thobhani S, Attree S, Boyd R, Kumarswami N, Noble J, Szymanski M, Porter RA. Bioconjugation and characterisation of gold colloid-labelled proteins. J Immunol Methods. 2010;356(1–2):60–9.
Li Q, Liu L, Chen W, Peng C, Wang L, Xu C. Gold nanoparticle-based immunochromatographic assay for the detection of 7-aminoclonazepam in urine. Int J Environ Anal Chem. 2009;89(4):261–8.
Li H, Sun B, Chen T. Detection of clothianidin residues in cucumber and apple juice using lateral-flow immunochromatographic assay. Food Agric Immunol. 2019;30(1):1112–22.
Song S, Suryoprabowo S, Liu L, Kuang H, Xu L, Ma W, Wu X. Development of monoclonal antibody-based colloidal gold immunochromatographic assay for analysis of halofuginone in milk. Food Agric Immunol. 2019;30(1):112–22.
Chen Z, Wu X, Xu L, Liu L, Kuang H, Cui G. Development of immunocolloidal strip for rapid detection of pyrimethanil. Food Agric Immunol. 2019;30(1):1239–52.
Zhang X, Liu L, Cui G, Song S, Kuang H, Xu C. Preparation of an anti-isoprocarb monoclonal antibody and its application in developing an immunochromatographic strip assay. Biomed Chromatogr. 2019;33(11):e4660.
Tian Z, Liu LQ, Peng C, Chen Z, Xu C. A new development of measurement of 19-nortestosterone by combining immunochromatographic strip assay and ImageJ software. Food Agric Immunol. 2009;20(1):1–10.
Shrivas K, Kant T, Karbhal I, Kurrey R, Sahu B, Sinha D, et al. Smartphone coupled with paper-based chemical sensor for on-site determination of iron (III) in environmental and biological samples. Anal Bioanal Chem. 2020;412(7):1573–83.
Li Q, Ren S, Peng Y, Lv Y, Wang W, Wang Z, et al. A colorimetric strip for rapid detection and real-time monitoring of histamine in fish based on self-assembled PDA vesicles. Anal Chem. 2019;92(1):1611–7.
Urban GL, Sultan F. The case for ‘Benevolent’ mobile apps. MIT Sloan Manag Rev. 2015;56(2):31.
Ricci L. Immersive media and branding: how being a brand will change and expand in the age of true immersion. In: Handbook of research on the global impacts and roles of immersive media: IGI Global; 2020. pp. 393–414.
Liu X, Xiang JJ, Tang Y, Zhang XL, Fu QQ, Zou JH, et al. Colloidal gold nanoparticle probe-based immunochromatographic assay for the rapid detection of chromium ions in water and serum samples. Anal Chim Acta. 2012;745:99–105. https://doi.org/10.1016/j.aca.2012.06.029.
Bala R, Braun KM. Color-to-grayscale conversion to maintain discriminability. Electronic Imaging 2004. SPIE. 2003;5293:196–202.
Di Nardo F, Alladio E, Baggiani C, Cavalera S, Giovannoli C, Spano G, Anfossi L. Colour-encoded lateral flow immunoassay for the simultaneous detection of aflatoxin B1 and type-B fumonisins in a single test line. Talanta. 2019;192:288–94.
Li X, Wang J, Yi C, Jiang L, Wu J, Chen X, Shen X, Sun Y, Lei H. A smartphone-based quantitative detection device integrated with latex microsphere immunochromatography for on-site detection of zearalenone in cereals and feed. Sensors Actuators B Chem. 2019;290:170–9.
Siangdee N, Youngvises N. Smart sensor using cellulose-based material for TNT detection. Key Eng Mat. 2019;803:124–8.
Vance GF, Krzyszowska AJ. Monitoring dicamba and picloram movement and fate in the vadose zone for groundwater quality protection in Wyoming. The Center; 1994.
Ensminger MP, Budd R, Kelley KC, Goh KS. Pesticide occurrence and aquatic benchmark exceedances in urban surface waters and sediments in three urban areas of California, USA, 2008–2011. Environ Monit Assess. 2013;185(5):3697–710.
Grover R, Waite DT, Cessna AJ, Nicholaichuk W, Irvin DG, Kerr LA, Best K. Magnitude and persistence of herbicide residues in farm dugouts and ponds in the Canadian prairies. Environ Toxicol Chem. 1997;16(4):638–43.
Zeng L, Wu X, Liu L, Xu L, Kuang H, Xu C. Production of a monoclonal antibody for the detection of vitamin B 1 and its use in an indirect enzyme-linked immunosorbent assay and immunochromatographic strip. J Mater Chem B. 2020;8(9):1935–43.
Hu G, Gao S, Han X, Yang L. Comparison of immunochromatographic strips using colloidal gold, quantum dots, and upconversion nanoparticles for visual detection of norfloxacin in milk samples. Food Anal Methods. 2020;13:1069–77.
Zhou X, Wang M, Cheng A, Yang Q, Wu Y, Jia R, et al. Development of a simple and rapid immunochromatographic strip test for detecting duck plague virus antibodies based on gI protein. J Virol Methods. 2020;277:113803.
Dong S, Liu Y, Zhang X, Xu C, Liu X, Zhang C. Development of an immunochromatographic assay for the specific detection of Bacillus thuringiensis (Bt) Cry1Ab toxin. Anal Biochem. 2019;567:1–7.
Qiuyan T, Yunlong W. Practical techniques of immunodiagnostic reagents. China Ocean Press; 2009.
Chenggang S, Suqing Z, ZHANG K, Guobao H, Zhenyu Z. Preparation of colloidal gold immunochromatography strip for detection of methamidophos residue. J Environ Sci. 2008;20(11):1392–7.
Xiulan S, Xiaolian Z, Jian T, Zhou J, Chu F. Preparation of gold-labeled antibody probe and its use in immunochromatography assay for detection of aflatoxin B1. Int J Food Microbiol. 2005;99(2):185–94.
Hu W, Yan Z, Li H, Qiu J, Zhang D, Li P, et al. Development of a new colloidal gold immunochromatographic strip for rapid detecting subgroup A of avian leukosis virus using colloidal gold nanoparticles. Biochem Eng J. 2019;148:16–23.
Rong-Hwa S, Shiao-Shek T, Der-Jiang C, Yao-Wen H. Gold nanoparticle-based lateral flow assay for detection of staphylococcal enterotoxin B. Food Chem. 2010;118(2):462–6.
Ling S, Chen Q-A, Zhang Y, Wang R, Jin N, Pang J, Wang S. Development of ELISA and colloidal gold immunoassay for tetrodotoxin detetcion based on monoclonal antibody. Biosens Bioelectron. 2015;71:256–60.
Sun C, Liu L, Song S, Kuang H, Xu C. Development of a highly sensitive ELISA and immunochromatographic strip to detect pentachlorophenol. Food Agric Immunol. 2016;27(5):689–99.
Cho YA, Kim YJ, Hammock BD, Lee YT, Lee H-S. Development of a microtiter plate ELISA and a dipstick ELISA for the determination of the organophosphorus insecticide fenthion. J Agric Food Chem. 2003;51(27):7854–60.
Jin Y, Jang J-W, Han C-H, Lee M-H. Development of ELISA and immunochromatographic assay for the detection of gentamicin. J Agric Food Chem. 2005;53(20):7639–43.
Health & Human Sciences Research Institute. The Pesticide Properties DataBase (PPDB). Agriculture & Environment Research Unit (AERU), University of Hertfordshire. 2013. http://hdl.handle.net/2299/15375. Accessed 15 Mar 2020.
Phillips PJ, Bode RW. Pesticides in surface water runoff in south-eastern New York State, USA: seasonal and stormflow effects on concentrations. Pest Manag Sci. 2004;60(6):531–43.
Hill BD, Harker KN, Hasselback P, Inaba DJ, Byers SD, Moyer JR. Herbicides in Alberta rainfall as affected by location, use and season: 1999 to 2000. Water Qual Res J. 2002;37(3):515–42.
Willett CD, Grantz EM, Lee JA, Thompson MN, Norsworthy JK. Soybean response to dicamba in irrigation water under controlled environmental conditions. Weed Sci. 2019;67(3):354–60.
Funding
This work was financially supported by the National Institute of Environmental Health Science Superfund Research Program (P42ES004699), the National Nature Science Foundation of China (No. 31871981), Graduate Research and Innovation Program of Hebei (CXZZBS2019099), the National Nature Science Foundation of Hebei (No. C2020204116) and the National Academy of Sciences (NAS, Subaward no. 2000009144). The article is derived from the subject data funded in part by NAS and USAID and opinions, findings, conclusions, or recommendations expressed in this article are those of the authors alone and do not necessarily reflect the views of USAID or NAS.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures involving animals were approved and performed in accordance with the relevant protective and administrative guidelines for laboratory animals of China.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qi, M., Huo, J., Li, Z. et al. On-spot quantitative analysis of dicamba in field waters using a lateral flow immunochromatographic strip with smartphone imaging. Anal Bioanal Chem 412, 6995–7006 (2020). https://doi.org/10.1007/s00216-020-02833-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02833-z