Abstract
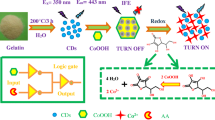
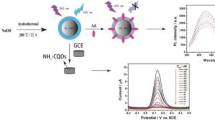
We present a facile strategy for highly sensitive and selective determination of copper(II) ions and vitamin C (ascorbic acid, AA) using new amino-terminated nitrogen-doped carbon quantum dots (CQDs) synthesized from melamine as the carbon and nitrogen source by the hydrothermal method. The CQDs have superior optical features, including a pH-sensitive photoinduced electron transfer process. The CQDs form a complex with Cu2+ ions, leading to the development of naked-eye, colorimetric, and fluorometric determination. AA reduces the Cu2+ ions to Cu+ ions, which cannot form the complex. Thus the absorbance and fluorescence of the CQDs are recovered by addition of AA because of dissociation of the complex into Cu+ and CQD. The in situ generation of reactive oxygen species when AA is added to Cu–CQD complexes in the presence of dissolved oxygen leads to the sensitive determination of AA, proposed on CQDs for the first time. The in situ generation of reactive oxygen species was confirmed by a fluorescence method using a hydroxyl radical indicator (i.e., coumarin). This novel “turn-off/turn-on” sensing approach using amine-functionalized CQDs is potentially applicable to determining the concentration of Cu2+ ions and AA in the areas of materials chemistry, nanobiomedicine, nanobiotechnology, and bioengineering because of its high sensitivity, high selectivity, low cost, simple naked-eye readout, and good linearity.

Graphical abstract







Similar content being viewed by others
References
Cayuela A, Soriano ML, Carrillo-Carrión C, Valcárcel M. Semiconductor and carbon-based fluorescent nanodots: the need for consistency. Chem Commun. 2016;52:1311–26. https://doi.org/10.1039/C5CC07754K.
Bianco A, Cheng H-M, Enoki T, Gogotsi Y, Hurt RH, Koratkar N, et al. All in the graphene family – a recommended nomenclature for two-dimensional carbon materials. Carbon N Y. 2013;65:1–6. https://doi.org/10.1016/j.carbon.2013.08.038.
Wang W, Yu JC, Shen Z, Chan DKL, Gu T. g-C3N4 quantum dots: direct synthesis, upconversion properties and photocatalytic application. Chem Commun. 2014;50:10148–50. https://doi.org/10.1039/C4CC02543A.
Kalaiyarasan G, Joseph J. Cholesterol derived carbon quantum dots as fluorescence probe for the specific detection of hemoglobin in diluted human blood samples. Mater Sci Eng C. 2019;94:580–6. https://doi.org/10.1016/j.msec.2018.10.007.
Kalaiyarasan G, Joseph J. Determination of vitamin B12 via pH-dependent quenching of the fluorescence of nitrogen doped carbon quantum dots. Microchim Acta. 2017;184:3883–91. https://doi.org/10.1007/s00604-017-2421-y.
Jung S, Chen X. Quantum dot-dye conjugates for biosensing, imaging, and therapy. Adv Healthc Mater. 2018;7:1800252. https://doi.org/10.1002/adhm.201800252.
Ku SH, Lee M, Park CB. Carbon-based nanomaterials for tissue engineering. Adv Healthc Mater. 2013;2:244–60. https://doi.org/10.1002/adhm.201200307.
Teradal NL, Jelinek R. Carbon nanomaterials in biological studies and biomedicine. Adv Healthc Mater. 2017;6:1700574. https://doi.org/10.1002/adhm.201700574.
Liu Z, Chen X, Zhang X, Gooding JJ, Zhou Y. Carbon-quantum-dots-loaded mesoporous silica nanocarriers with pH-switchable zwitterionic surface and enzyme-responsive pore-cap for targeted imaging and drug delivery to tumor. Adv Healthc Mater. 2016;5:1401–7. https://doi.org/10.1002/adhm.201600002.
Scheiber I, Dringen R, Mercer JFB. Copper: effects of deficiency and overload. Met Ions Life Sci. 2013;13:359–87. https://doi.org/10.1007/978-94-007-7500-8-11.
Halfdanarson TR, Kumar N, Li C-Y, Phyliky RL, Hogan WJ. Hematological manifestations of copper deficiency: a retrospective review. Eur J Haematol. 2008;80:523–31. https://doi.org/10.1111/j.1600-0609.2008.01050.x.
Bandmann O, Weiss KH, Kaler SG. Wilson's disease and other neurological copper disorders. The Lancet. 2015;14(1):103–13. https://doi.org/10.1016/S1474-4422(14)70190-5.
Bagheri S, Squitti R, Haertlé T, Siotto M, Saboury AA. Role of copper in the onset of Alzheimer’s disease compared to other metals. Front Aging Neurosci. 2018;9:1–15. https://doi.org/10.3389/fnagi.2017.00446.
Smallwood RA, Williams HA, Rosenoer VM, Sherlock S. Liver-copper levels in liver disease: studies using neutron activation analysis. Lancet. 1968;292:1310–3. https://doi.org/10.1016/S0140-6736(68)91814-X.
Sondheimer JH, Mahajan SK, Rye DL, Abu-Hamdan DK, Migdal SD, Prasad AS, et al. Elevated plasma copper in chronic renal failure. Am J Clin Nutr. 1988;47:896–9. https://doi.org/10.1093/ajcn/47.5.896.
Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17:1328. https://doi.org/10.3390/ijms17081328.
Hemilä H. Vitamin C supplementation and the common cold—was Linus Pauling right or wrong? Int J Vitam Nutr Res. 1997;67:329–35.
Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13:1007–24. https://doi.org/10.1096/fasebj.13.9.1007.
Hepel M, Stobiecka M, Peachey J, Miller J. Intervention of glutathione in pre-mutagenic catechol-mediated DNA damage in the presence of copper(II) ions. Mutat Res Mol Mech Mutagen. 2012;735:1–11. https://doi.org/10.1016/j.mrfmmm.2012.05.005.
Shao H, Xu D, Ding Y, Hong X, Liu Y. An “off-on” colorimetric and fluorometric assay for Cu(II) based on the use of NaYF4:Yb(III),Er(III) upconversion nanoparticles functionalized with branched polyethyleneimine. Microchim Acta. 2018;185:211. https://doi.org/10.1007/s00604-018-2740-7.
Chen X, Lu Q, Liu D, Wu C, Liu M, Li H, et al. Highly sensitive and selective determination of copper(II) based on a dual catalytic effect and by using silicon nanoparticles as a fluorescent probe. Microchim Acta. 2018;185:188. https://doi.org/10.1007/s00604-018-2720-y.
Wang J, Peng X, Li D, Jiang X, Pan Z, Chen A, et al. Ratiometric ultrasensitive fluorometric detection of ascorbic acid using a dually emitting CdSe@SiO2@CdTe quantum dot hybrid. Microchim Acta. 2018;185:42. https://doi.org/10.1007/s00604-017-2557-9.
Cui W, Wang Y, Yang D, Du J. Fluorometric determination of ascorbic acid by exploiting its deactivating effect on the oxidase–mimetic properties of cobalt oxyhydroxide nanosheets. Microchim Acta. 2017;184:4749–55. https://doi.org/10.1007/s00604-017-2525-4.
Kim D. Bin, Hong JM, Chang SK (2017) Colorimetric determination of Cu2+ ions with a desktop scanner using silica nanoparticles via formation of a quinonediimine dye. Sens Actuators B. 2017;252:537–43. https://doi.org/10.1016/j.snb.2017.06.033.
Xu L, Wei S, Diao Q, Ma P, Liu X, Sun Y, et al. Sensitive and selective rhodamine-derived probes for fluorometric sensing of pH and colorimetric sensing of Cu2+. Sens Actuators B. 2017;246:395–401. https://doi.org/10.1016/j.snb.2017.02.093.
Liu Y, Ding D, Zhen Y, Guo R. Amino acid-mediated ‘turn-off/turn-on’ nanozyme activity of gold nanoclusters for sensitive and selective detection of copper ions and histidine. Biosens Bioelectron. 2017;92:140–6. https://doi.org/10.1016/j.bios.2017.01.036.
Li R, An H, Huang W, He Y. Molybdenum oxide nanosheets meet ascorbic acid: tunable surface plasmon resonance and visual colorimetric detection at room temperature. Sens Actuators B. 2018;259:59–63. https://doi.org/10.1016/j.snb.2017.12.058.
Ji D, Du Y, Meng H, Zhang L, Huang Z, Hu Y, et al. A novel colorimetric strategy for sensitive and rapid sensing of ascorbic acid using cobalt oxyhydroxide nanoflakes and 3,3′,5,5′-tetramethylbenzidine. Sens Actuators B. 2018;256:512–9. https://doi.org/10.1016/j.snb.2017.10.070.
Razmi H, Harasi M. Voltammetric behavior and amperometric determination of ascorbic acid at cadmium pentacyanonitrosylferrate film modified GC electrode. Int J Electrochem Sci. 2008;3:82–95.
Kalaiyarasan G, Narendra Kumar AV, Sivakumar C, Joseph J. Photoluminescence of oligomers of aniline-2-sulfonic acid formed in the presence of AuCl4 − and sodium citrate: application in the optical detection of hemoglobin. Sens Actuators B. 2015;209:883–8. https://doi.org/10.1016/j.snb.2014.12.041.
Kalaiyarasan G, Kathiresan A, Joseph J. Melamine dependent fluorescence of glutathione protected gold nanoclusters and ratiometric quantification of melamine in commercial cow milk and infant formula. Appl Surf Sci. 2017;420:963–9. https://doi.org/10.1016/j.apsusc.2017.05.193.
Li G, Lian Z, Wang W, Zhang D, Li H. Nanotube-confinement induced size-controllable g-C3N4 quantum dots modified single-crystalline TiO2 nanotube arrays for stable synergetic photoelectrocatalysis. Nano Energy. 2016;19:446–54. https://doi.org/10.1016/j.nanoen.2015.10.011.
Liu F, Jiang Y, Fan C, Zhang L, Hua Y, Zhang C, et al. Fluorimetric and colorimetric analysis of total iron ions in blood or tap water using nitrogen-doped carbon dots with tunable fluorescence. New J Chem. 2018;42:9676–83. https://doi.org/10.1039/C8NJ00711J.
Hepel M, Blake D, McCabe M, Stobiecka M, Coopersmith K. Assembly of gold nanoparticles induced by metal ions. ACS Symp Ser. 2012;1112:207–40. https://doi.org/10.1021/bk-2012-1112.ch008.
Yang M, Wang J, Zhou F. Functional nanoparticles for bioanalysis, nanomedicine, and bioelectronic devices. Vol. 1. Washington: American Chemical Society; 2012.
Huang S, Qiu H, Zhu F, Lu S, Xiao Q. Graphene quantum dots as on-off-on fluorescent probes for chromium(VI) and ascorbic acid. Microchim Acta. 2015;182:1723–31. https://doi.org/10.1007/s00604-015-1508-6.
Zhao L, Xin X, Ding P, Song A, Xie Z, Shen J, et al. Fluorescent oligomer as a chemosensor for the label-free detection of Fe3+ and dopamine with selectivity and sensitivity. Anal Chim Acta. 2016;926:99–106. https://doi.org/10.1016/j.aca.2016.04.038.
Anjana RR, Anjali Devi JS, Jayasree M, Aparna RS, Aswathy B, Praveen GL, et al. S,N-doped carbon dots as a fluorescent probe for bilirubin. Microchim Acta. 2018;185:1–11. https://doi.org/10.1007/s00604-017-2574-8.
Xu H, Zhou S, Liu J, Wei Y. Nanospace-con fined preparation of uniform nitrogen-doped graphene quantum dots for highly selective fluorescence dual-function determination. RSC Adv. 2018;8:5500–8. https://doi.org/10.1039/C7RA13001E.
Liu JJ, Chen ZT, Tang DS, Wang YB, Kang LT, Yao JN. Graphene quantum dots-based fluorescent probe for turn-on sensing of ascorbic acid. Sens Actuators B. 2015;212:214–9. https://doi.org/10.1016/j.snb.2015.02.019.
Li X, Zhang S, Kulinich SA, Liu Y, Zeng H. Engineering surface states of carbon dots to achieve controllable luminescence for solid-luminescent composites and sensitive Be2+ detection. Sci Rep. 2015;4:4976. https://doi.org/10.1038/srep04976.
Xu M, Xu S, Yang Z, Shu M, He G, Huang D, et al. Hydrophilic and blue fluorescent N-doped carbon dots from tartaric acid and various alkylol amines under microwave irradiation. Nanoscale. 2015;7:15915–123. https://doi.org/10.1039/C5NR04209G.
Kalaiyarasan G, Hemlata C, Joseph J. Fluorescence turn-on, specific detection of cystine in human blood plasma and urine samples by nitrogen-doped carbon quantum dots. ACS Omega. 2019;4:1007–14. https://doi.org/10.1021/acsomega.8b03187.
Wang J, Zhang P, Huang C, Liu G, Leung KC-F, Wáng YXJ. High performance photoluminescent carbon dots for in vitro and in vivo bioimaging: effect of nitrogen doping ratios. Langmuir. 2015;31:8063–73. https://doi.org/10.1021/acs.langmuir.5b01875.
Hu R, Li L, Jin WJ. Controlling speciation of nitrogen in nitrogen-doped carbon dots by ferric ion catalysis for enhancing fluorescence. Carbon N Y. 2017;111:133–41. https://doi.org/10.1016/j.carbon.2016.09.038.
Paul BK, Guchhait N. Modulated photophysics of an ESIPT probe 1-hydroxy-2-naphthaldehyde within motionally restricted environments of liposome membranes having varying surface charges. J Phys Chem B. 2010;114:12528–40. https://doi.org/10.1021/jp1048138.
Ray D, Paul BK, Guchhait N. Effect of biological confinement on the photophysics and dynamics of a proton-transfer phototautomer: an exploration of excitation and emission wavelength-dependent photophysics of the protein-bound drug. Phys Chem Chem Phys. 2012;14:12182–92. https://doi.org/10.1039/c2cp41292f.
Paul BK, Guchhait N. Morphological transition of the host-structure influences solvent-relaxation: A wavelength-selective fluorescence exploration through environment-sensitive intramolecular charge transfer photophysics. Spectrochim Acta Part A. 2011;81:590–7. https://doi.org/10.1016/j.saa.2011.06.056.
Sun YP, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc. 2006;128:7756–7. https://doi.org/10.1021/ja062677d.
Li L, Li L, Wang C, Liu K, Zhu R, Qiang H, et al. Synthesis of nitrogen-doped and amino acid-functionalized graphene quantum dots from glycine, and their application to the fluorometric determination of ferric ion. Microchim Acta. 2014;182:763–70. https://doi.org/10.1007/s00604-014-1383-6.
Park YR, Jeong HY, Seo YS, Choi WK, Hong YJ. Quantum-dot light-emitting diodes with nitrogen-doped carbon nanodot hole transport and electronic energy transfer layer. Sci Rep. 2017;7:46422. https://doi.org/10.1038/srep46422.
Ding H, Yu S-B, Wei J-S, Xiong H-M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano. 2016;10:484–91. https://doi.org/10.1021/acsnano.5b05406.
Ding W, Wei Z, Chen S, Qi X, Yang T, Hu J, et al. Space-confinement-induced synthesis of pyridinic- and pyrrolic-nitrogen-doped graphene for the catalysis of oxygen reduction. Angew Chemie Int Ed. 2013;52:11755–9. https://doi.org/10.1002/anie.201303924.
Dong MM, He C, Zhang WX. A tunable and sizable bandgap of a g-C3N4/graphene/g-C3N4 sandwich heterostructure: a van der Waals density functional study. J Mater Chem C. 2017;5:3830–7. https://doi.org/10.1039/C7TC00386B.
Shen L, Chen M, Hu L, Chen X, Wang J. Growth and stabilization of silver nanoparticles on carbon dots and sensing application. Langmuir. 2013;29:16135–40. https://doi.org/10.1021/la404270w.
Jin J-C, Xu Z-Q, Dong P, Lai L, Lan J-Y, Jiang F-L, et al. One-step synthesis of silver nanoparticles using carbon dots as reducing and stabilizing agents and their antibacterial mechanisms. Carbon N Y. 2015;94:129–41. https://doi.org/10.1016/J.CARBON.2015.05.084.
De B, Kuila T, Kim NH, Lee JH. Carbon dot stabilized copper sulphide nanoparticles decorated graphene oxide hydrogel for high performance asymmetric supercapacitor. Carbon N Y. 2017;122:247–57. https://doi.org/10.1016/J.CARBON.2017.06.076.
Liu R, Liu J, Kong W, Huang H, Han X, Zhang X, et al. Adsorption dominant catalytic activity of a carbon dots stabilized gold nanoparticles system. Dalton Trans. 2014;43:10920. https://doi.org/10.1039/c4dt00630e.
Pollock N, Fowler G, Twyman LJ, McArthur SL. Synthesis and characterization of immobilized PAMAM dendrons. Chem Commun. 2007:2482. https://doi.org/10.1039/b701550j.
Cross JB, Currier RP, Torraco DJ, Vanderberg LA, Wagner GL, Gladen PD. Killing of Bacillus spores by aqueous dissolved oxygen, ascorbic acid, and copper ions. Appl Environ Microbiol. 2003;69:2245–52. https://doi.org/10.1128/AEM.69.4.2245-2252.2003.
Louit G, Foley S, Cabillic J, Coffigny H, Taran F, Valleix A, et al. The reaction of coumarin with the OH radical revisited: hydroxylation product analysis determined by fluorescence and chromatography. Radiat Phys Chem. 2005;72:119–24. https://doi.org/10.1016/j.radphyschem.2004.09.007.
Huang H, Chen R, Ma J, Yan L, Zhao Y, Wang Y, et al. Graphitic carbon nitride solid nanofilms for selective and recyclable sensing of Cu2+ and Ag+ in water and serum. Chem Commun. 2014;50:15415–8. https://doi.org/10.1039/c4cc06659f.
Kalaiyarasan G, Narendra Kumar AV, Sivakumar C, Joseph J. Electro-generated reactive oxygen species at Au surface as an indicator to explore glutathione redox chemistry and quantification. Electrochem Commun. 2015;56:29–33. https://doi.org/10.1016/j.elecom.2015.03.021.
Acknowledgements
GK thanks joint CSIR-UGC, India, for the award of a senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
The study was approved by the ethics committee of the CSIR Central Electrochemical Research Institute health center and was performed in accordance with ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 801 kb)
Rights and permissions
About this article
Cite this article
Kalaiyarasan, G., Joseph, J. Efficient dual-mode colorimetric/fluorometric sensor for the detection of copper ions and vitamin C based on pH-sensitive amino-terminated nitrogen-doped carbon quantum dots: effect of reactive oxygen species and antioxidants. Anal Bioanal Chem 411, 2619–2633 (2019). https://doi.org/10.1007/s00216-019-01710-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01710-8