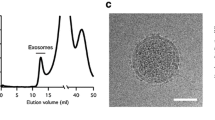
Abstract
The development of a sensitive and specific detection platform for exosomes is highly desirable as they are believed to transmit vital tumour-specific information (mRNAs, microRNAs, and proteins) to remote cells for secondary metastasis. Herein, we report a simple method for the real-time and label-free detection of clinically relevant exosomes using a surface plasmon resonance (SPR) biosensor. Our method shows high specificity in detecting BT474 breast cancer cell–derived exosomes particularly from complex biological samples (e.g. exosome spiked in serum). This approach exhibits high sensitivity by detecting as low as 8280 exosomes/μL which may potentially be suitable for clinical analysis. We believe that this label-free and real-time method along with the high specificity and sensitivity may potentially be useful for clinical settings.




Similar content being viewed by others
References
Grasso L, Wyss R, Weidenauer L, Thampi A, Demurtas D, Prudent M, et al. Molecular screening of cancer-derived exosomes by surface plasmon resonance spectroscopy. Anal Bioanal Chem. 2015;407:5425–32. https://doi.org/10.1007/s00216-015-8711-5.
Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–68. https://doi.org/10.1111/j.1600-0854.2011.01225.x.
van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. https://doi.org/10.1124/pr.112.005983.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9.
Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. https://doi.org/10.1038/nri2567.
Principe S, Hui AB-Y, Bruce J, Sinha A, Liu F-F, Kislinger T. Tumor-derived exosomes and microvesicles in head and neck cancer: implications for tumor biology and biomarker discovery. Proteomics. 2013;13:1608–23. https://doi.org/10.1002/pmic.201200533.
Fais S, O’Driscoll L, Borras FE, Buzas E, Camussi G, Cappello F, et al. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano. 2016;10:3886–99. https://doi.org/10.1021/acsnano.5b08015.
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6.
Brinton LT, Sloane HS, Kester M, Kelly KA. Formation and role of exosomes in cancer. Cell Mol Life Sci. 2015;72:659–71. https://doi.org/10.1007/s00018-014-1764-3.
Lane RE, Korbie D, Hill MM, Trau M. Extracellular vesicles as circulating cancer biomarkers: opportunities and challenges. Clin Transl Med. 2018;7:14. https://doi.org/10.1186/s40169-018-0192-7.
Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;30:1–29. https://doi.org/10.1002/0471143030.cb0322s30.
Wubbolts R, Leckie RS, Veenhuizen PTM, Schwarzmann G, Möbius W, Hoernschemeyer J, et al. Proteomic and biochemical analyses of human B cell-derived exosomes: potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278:10963–72. https://doi.org/10.1074/jbc.M207550200.
Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270:211–26.
Zhou H, Yuen PST, Pisitkun T, Gonzales PA, Yasuda H, Dear JW, et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006;69:1471–6. https://doi.org/10.1016/j.polymdegradstab.2005.10.005.
Ding M, Wang C, Lu X, Zhang C, Zhou Z, Chen X, et al. Comparison of commercial exosome isolation kits for circulating exosomal microRNA profiling. Anal Bioanal Chem. 2018;410:3805–14. https://doi.org/10.1007/s00216-018-1052-4.
Vaidyanathan R, Naghibosadat M, Rauf S, Korbie D, Carrascosa LG, Shiddiky MJA, et al. Detecting exosomes specifically: a multiplexed device based on alternating current electrohydrodynamic induced nanoshearing. Anal Chem. 2014;86:11125–32.
Henderson RD, Guijt RM, Andrewartha L, Lewis TW, Rodemann T, Henderson A, et al. Lab-on-a-Chip device with laser-patterned polymer electrodes for high voltage application and contactless conductivity detection. Chem Commun. 2012;48:9287–9. https://doi.org/10.1039/c2cc33693f.
Wang Z, Wu H, Fine D, Schmulen J, Hu Y, Godin B, et al. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip. 2013;13:2879–82. https://doi.org/10.1039/c3lc41343h.
Moschou D, Greathead L, Pantelidis P, Kelleher P, Morgan H, Prodromakis T. Amperometric IFN-γ immunosensors with commercially fabricated PCB sensing electrodes. Biosens Bioelectron. 2016;86:805–10. https://doi.org/10.1016/j.bios.2016.07.075.
Pechlivanidis NG, Papadimitriou KI, Evans D, Vasilakis N, Prodromakis T. Towards a smartphone-aided electronic ELISA for real-time electrochemical monitoring. In: 2017 IEEE Int. Symp. Circuits Syst. 2017: pp. 1–4. https://doi.org/10.1109/ISCAS.2017.8050616.
Evans D, Papadimitriou KI, Greathead L, Vasilakis N, Pantelidis P, Kelleher P, et al. An assay system for point-of-care diagnosis of tuberculosis using commercially manufactured PCB technology. Sci Rep. 2017;7:1–10. https://doi.org/10.1038/s41598-017-00783-8.
Wuethrich A, Howard CB, Trau M. Geometric optimisation of electrohydrodynamic fluid flows for enhanced biosensing. Microchem J. 2018;137:231–7. https://doi.org/10.1016/j.microc.2017.10.012.
Wuethrich A, Sina AAI, Ahmed M, Lina T-Y, Carrascosa LG, Trau M. Interfacial nano-mixing in a miniaturised platform enables signal enhancement and in situ detection of cancer biomarkers. Nanoscale. 2018;10:10884–90. https://doi.org/10.1039/c7nr09496e.
Im H, Shao H, Il PY, Peterson VM, Castro CM, Weissleder R, et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat Biotechnol. 2014;32:490–5. https://doi.org/10.1038/nbt.2886.
Zhu L, Wang K, Cui J, Liu H, Bu X, Ma H, et al. Label-free quantitative detection of tumor-derived exosomes through surface plasmon resonance imaging. Anal Chem. 2014;86:8857–64. https://doi.org/10.1021/ac5023056.
Di Noto G, Bugatti A, Zendrini A, Mazzoldi EL, Montanelli A, Caimi L, et al. Merging colloidal nanoplasmonics and surface plasmon resonance spectroscopy for enhanced profiling of multiple myeloma-derived exosomes. Biosens Bioelectron. 2016;77:518–24. https://doi.org/10.1016/j.bios.2015.09.061.
Chen J, Park B. Label-free screening of foodborne Salmonella using surface plasmon resonance imaging. Anal Bioanal Chem. 2018;410:5455–64. https://doi.org/10.1007/s00216-017-0810-z.
Tai Y-H, Fu P-H, Lee K-L, Wei P-K. Spectral imaging analysis for ultrasensitive biomolecular detection using gold-capped nanowire arrays. Sensors. 2018;18:2181. https://doi.org/10.3390/s18072181.
Bustos RH, Zapata C, Esteban E, García JC, Jáuregui E, Jaimes D. Label-free quantification of anti-TNF-α in patients treated with adalimumab using an optical biosensor. Sensors. 2018;18:691. https://doi.org/10.3390/s18030691.
Liu C, Zeng X, An Z, Yang Y, Eisenbaum M, Gu X, et al. Sensitive detection of exosomal proteins via a compact surface plasmon resonance biosensor for cancer diagnosis. ACS Sensors. 2018;3:1471–9. https://doi.org/10.1021/acssensors.8b00230.
Picciolini S, Gualerzi A, Vanna R, Sguassero A, Gramatica F, Bedoni M, et al. Detection and characterization of different brain-derived subpopulations of plasma exosomes by surface plasmon resonance imaging. Anal Chem. 2018;90:8873–80. https://doi.org/10.1021/acs.analchem.8b00941.
Rupert DLM, Shelke GV, Emilsson G, Claudio V, Block S, Lässer C, et al. Dual-wavelength surface plasmon resonance for determining the size and concentration of sub-populations of extracellular vesicles. Anal Chem. 2016;88:9980–8. https://doi.org/10.1021/acs.analchem.6b01860.
Duraichelvan R, Srinivas B, Badilescu S, Ouellette R, Ghosh A, Packirisamy M. Exosomes detection by a label-free localized surface plasmonic resonance method. ECS Trans. 2016;75:11–7. https://doi.org/10.1093/glycob/7.1.79.
Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012;18:1835–40. https://doi.org/10.1038/nm.2994.
Sina AAI, Vaidyanathan R, Dey S, Carrascosa LG, Shiddiky MJA, Trau M. Real time and label free profiling of clinically relevant exosomes. Sci Rep. 2016;6:30460. https://doi.org/10.1038/srep30460.
Vasilakis N, Papadimitriou KI, Evans D, Morgan H, Prodromakis T. The Lab-on-PCB framework for affordable, electronic-based point-of-care diagnostics: from design to manufacturing. 2016 IEEE Healthc Innov Point-of-Care Technol Conf HI-POCT. 2016;2016:126–9. https://doi.org/10.1109/HIC.2016.7797713.
Roberts GS, Yu S, Zeng Q, Chan LCL, Anderson W, Colby AH, et al. Tunable pores for measuring concentrations of synthetic and biological nanoparticle dispersions. Biosens Bioelectron. 2012;31:17–25. https://doi.org/10.1016/j.bios.2011.09.040.
Carlsson J, Nordgren H, Sjöström J, Wester K, Villman K, Bengtsson NO, et al. HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review. Br J Cancer. 2004;90:2344–8. https://doi.org/10.1038/sj.bjc.6601881.
Coleman BM, Hanssen E, Lawson VA, Hill AF. Prion-infected cells regulate the release of exosomes with distinct ultrastructural features. FASEB J. 2012;26:4160–73. https://doi.org/10.1096/fj.11-202077.
De Vrij J, Maas SLN, Van Nispen M, Sena-Esteves M, Limpens RWA, Koster AJ, et al. Quantification of nanosized extracellular membrane vesicles with scanning ion occlusion sensing. Nanomedicine. 2013;8:1443–58. https://doi.org/10.2217/nnm.12.173.
Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:1–14. https://doi.org/10.1186/1471-2164-14-319.
Cheng Z, Wang Z, Gillespie DE, Lausted C, Zheng Z, Yang M, et al. Plain silver surface plasmon resonance for microarray application. Anal Chem. 2015;87:1466–9. https://doi.org/10.1021/ac504110t.
Wang Z, Cheng Z, Singh V, Zheng Z, Wang Y, Li S, et al. Stable and sensitive silver surface plasmon resonance imaging sensor using trilayered metallic structures. Anal Chem. 2014;86:1430–6. https://doi.org/10.1021/ac402126k.
Acknowledgements
We acknowledge the support from the Australian National Fabrication Facility (ANFF) for SPR chip fabrication, and Center for Microscopy and Microanalysis (CMM) for cryo-TEM facility.
Funding
This study received funding from the Australian Research Council (DP180102868). AW received funding from the University of Queensland for the Development Fellowship (UQFEL1831057).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 480 kb)
Rights and permissions
About this article
Cite this article
Sina, A.A.I., Vaidyanathan, R., Wuethrich, A. et al. Label-free detection of exosomes using a surface plasmon resonance biosensor. Anal Bioanal Chem 411, 1311–1318 (2019). https://doi.org/10.1007/s00216-019-01608-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01608-5