Abstract
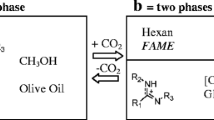
We describe a new use of switchable-polarity solvents for the simultaneous derivatization and extraction of triacylglycerols from vegetable oils before gas-chromatographic analysis. Different equimolecular mixtures of the commercially available amidine 1,8-diazabicyclo[5.4.0]undec-7-ene and n-alkyl alcohols were tested. Triolein was used as a model compound. Very good results were achieved by using butanol (recovery of butyl oleate was 89 ± 4 %). The procedure was applied for the characterization of the fatty acid profile of different vegetable oils. No statistically significant differences from the results obtained with the application of two traditional methods were evidenced. Moreover, the use of switchable-polarity solvents showed many advantages: owing to the basicity of the amidines, no catalyst was required; the transterification reaction was conducted under mild conditions, one step and in situ; no particular matrix interferences were evidenced; the solvent was recovered.

Switchable polarity solvents perform the simultaneous extraction and transesterification of triacylglycerols from vegetable oils. The method represent a new in situ derivatization procedure for the gas chromatographic characterization of thir fatty acid profiles.


Similar content being viewed by others
References
Eckey EW (1954) Vegetable fats and oils. Reinhold Publishing, New York, p 51
Dodds ED, McCoy MR, Rea LD, Kennish JM (2005) Lipids 40:419–428
Metcalfe LD, Schmitz AA (1961) Anal Chem 33:363–364
Morrison WR, Smith LM (1964) J Lipid Res 53:600–608
Carrapiso AI, García C (2000) Lipids 35:1167–1177
Marx F, Stender A (1997) Lipids 99:25–28
Craig BM, Tulloch AP, Murty NL (1963) J Am Oil Chem Soc 40:61–63
Taber DF, Gerstenhaber D, Zhao X (2006) Tetrahedron Lett 47:3065–3066
Hallmann C, van Aarssen BGK, Grice K (2008) J Chromatogr A 14:1198–1199
Woo KL, Kim JL (1999) J Chromatogr A 862:199–208
Christie WW (2003) Lipid analysis. Isolation, separation, identification and structural analysis of lipids, 3rd edn. The Oily Press, Bridgewater
Byrdwell WC (2001) Lipids 36:327–346
Bosque-Sendra JM, Cuadros-Rodriguez L, Ruiz-Samblas CA, de la Mata P (2012) Anal Chim Acta 724:1–11
Ruiz-Samblás C, Cuadros-Rodríguez L, González-Casado A, de Paula Rodríguez García F, de la Mata-Espinosa P, Bosque-Sendra JM (2011) Anal Bioanal Chem 399:2093–2103
Byrdwell WC, Emken EA (1995) Lipids 30:173–175
Kovacevic B, Maksic ZB (2001) Org Lett 3:1523–1526
Schuchardt U, Vargas RM, Gelbard G (1996) J Mol Catal A 109:37–44
Gelbard G, Vielfaure-Joly F (1998) Tetrahedron Lett 39:2743–2746
Venkat Reddy CR, Fetterly BM, Verkade JG (2007) Energy Fuel 21:2466–2472
Peter S, Weidner E (2007) Eur J Lipid Sci Technol 109:11–16
Schuchardt U, Lopes OC (1988) J Am Oil Chem Soc 65:1940–1941
Jessop PG, Heldebrant DJ, Xiaowang L, Eckert CA, Liotta CL (2005) Nature 436:1102
Phan L, Brown H, White J, Hodgson A, Jessop PG (2009) Green Chem 11:53–59
Samorì C, Torri C, Samorì G, Fabbri D, Galletti P, Guerrini F, Pistocchi R, Tagliavini E (2010) Bioresour Technol 101:3274–3279
Phan L, Chiu D, Heldebrant DJ, Huttenhower H, John E, Li X, Pollet P, Wang R, Eckert CA, Liotta CL, Jessop PG (2008) Ind Eng Chem Res 47:539–545
Joseph JD, Ackman RG (1992) J AOAC Int 75:488–506
Dourtoglou T, Stefanou E, Lalas S, Dourtoglou V, Poulosc C (2001) Analyst 126:1032–1036
Heldebrant DJ, Jessop PG, Thomas CJ, Eckert CA, Liotta CL (2005) J Org Chem 70:5335–5338
Rusch GM, Hoffmann GM, McConnell RF, Rinehart WE (1986) Toxicol Appl Pharmacol 83:69–78
Orgambide GG, Reusch NN, Dazzo FB (1993) J Bacteriol 17:4922–4926
Yurawecz MP, Molina AA, Mossoba M, Ku Y (1993) J Am Oil Chem Soc 70:1093–1099
Kodad O, Socias R (2008) J Agric Food Chem 56:4096–4101
Moayedi A, Rezaei K, Moini S, Keshavarz B (2011) J Am Oil Chem Soc 88:503–508
Abdallah A, Ahumada MH, Gradziel TM (1998) J Am Soc Hortic Sci 123:1029–1033
Belcadi-Haloui R, Zekhnini A, Hatimi A (2008) Acta Bot Gallica 155:301–305
Charrouf Z, Guillaume D (2008) Eur J Lipid Sci Technol 110:632–636
Azrina A, Lim PH, Amin I, Zulkhairi A (2009) J Food Agric Environ 7:256–262
Tan BK, Hamilton RJ, Berger KG (1981) J Am Oil Chem Soc 58:1–5
Ayorinde FO, Garvin K, Saeed K (2000) Rapid Commun Mass Spectrom 14:608–615
Acknowledgments
We thank Ilaria Piga Serra, Melody Rose DeSanto, and our students Andrea Bosiso and Javier Gomez for their collaboration. This work was supported by University of Milan-Bicocca Far 2012. Two anonymous reviewers are also thanked for comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saliu, F., Orlandi, M. In situ alcoholysis of triacylglycerols by application of switchable-polarity solvents. A new derivatization procedure for the gas-chromatographic analysis of vegetable oils. Anal Bioanal Chem 405, 8677–8684 (2013). https://doi.org/10.1007/s00216-013-7190-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7190-9