Abstract
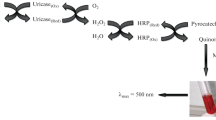
Peroxidase-catalysed reactions are used in a wide variety of analytical applications, most of them based on the final quantification of hydrogen peroxide. Clinical tests for glucose, cholesterol, creatine, creatinine or uric acid in blood or urine and enzyme-linked immunosorbent assays for pesticides, hepatitis or acquired immune deficiency syndrome are good examples of such applications. The most widely used and commercially available peroxidase for biotechnological processes and analytical applications is horseradish peroxidase followed, although in much lower proportion, by soybean peroxidase. The high commercial interest in peroxidases has led to the search for new sources of these enzymes. This work describes the analytical use of lentil plant peroxidase (LPP), which is a new peroxidase extracted from lentil plants (Lens culinaris Medikus); an abundant post-harvest agricultural waste in the area of Castilla y León (Spain). A procedure for the quantification of hydrogen peroxide in urine is first proposed using crude extract of lentil plant instead of the purified enzyme. This procedure is then applied to the determination of sarcosine; a natural amino acid that has attracted considerable interest in clinical diagnostics since urinary sarcosine was proposed and later questioned as a biomarker for prostate cancer. Under the action of sarcosine oxidase, sarcosine is oxidized by molecular oxygen to give glycine, formaldehyde and hydrogen peroxide that is quantified according to the previously proposed procedure. The limit of detection for both hydrogen peroxide and sarcosine is around 5 × 10−7 M. In the determination of sarcosine, the high selectivity of the overall enzymatic reaction, the simple sample treatment and instrumentation, the high-sample throughput and the use of LPP in the plant extract instead of the purified enzyme provide a rapid and inexpensive procedure with characteristics very suitable for routine analysis in a clinical laboratory.

Lentil plant of ‘Rubia de la Armuña’ (Lens culinaris Medikus)/Determination of sarcosine


Similar content being viewed by others
References
Ngo TT (2010) Peroxidase in chemical and biochemical analysis. Anal Lett 43:1572–1587
Hamid M, Khalil-ur-Rehman (2009) Potential applications of peroxidases. Food Chem 115:1177–1186
Regalado C, García-Almendárez BE, Duarte-Vázquez MA (2004) Biotechnological applications of peroxidases. Phytochem Rev 3:243–256
Screening, extraction and purification of peroxidases from green agricultural wastes: characterization and evaluation of some of their biotechnological applications. Project supported by Junta de Castilla y León (Spain) (Refs.: (SA129A07)/ITACYL SA-06-00-0)
Fatibello-Filho O, Vieira IC (2002) Uso analítico de tecidos e de extractos brutos vegetais como fonte enzimatica. Quim Nova 25:455–464
Fatibello-Filho O, Lupetti KO, Leite OD, Vieira IC (2007) Electrochemical biosensors based on vegetable tissues and crude extracts for environmental, food and pharmaceutical analysis. Compr Anal Chem 49:357–377
Moshides JS (1988) Enzymic determination of the free cholesterol fraction of high-density lipoprotein in plasma with use of 2,4,6-tribromo-3-hydroxybenzoic acid. Clin Chem 34:1799–1804
Nicell JA, Wright H (1997) A model of peroxidase activity with inhibition by hydrogen peroxide. Enzyme Microb Tech 21:302–310
Carvalho RH, Lemos F, Lemos MA, Vojinović V, Fonseca LP, Cabral JM (2006) Kinetic modelling of phenol co-oxidation using horseradish peroxidase. Bioprocess Biosyst Eng 29:99–108
TCI Product Literature (2011) Chromogenic peroxidase substrates, including hydrogen peroxide detection. http://www.tcieurope.eu/es/useful-info/product-lit/L3015E.pdf
Sreekumar A, Poisson LM, Rajendiran TM (2009) Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 457:910–915
Jentzmik F, Stephan C, Miller K, Schrader M, Erbersdobler A, Kristiansen G, Lein M, Jung K (2010) Sarcosine in urine after digital rectal examination fails as a marker in prostate cancer detection and identification of aggressive tumours. Eur Urol 58:12–18
Schalken JA (2010) Is urinary sarcosine useful to identify patients with significant prostate cancer? The trials and tribulations of biomarkers development. Eur Urol 58:19–21
Struys EA, Heijboer AC, Van Moorselaar J, Jackobs C, Blankenstein MA (2010) Serum sarcosine is not a marker for prostate cancer. Ann Clin Biochem 47:282–282
Jiang Y, Cheng X, Wang C, Ma Y (2010) Quantitative determination of sarcosine and related compounds in urinary samples by liquid chromatography with tandem mass spectrometry. Anal Chem 82:9022–0027
Issaq HJ, Veenstra TD (2011) Is sarcosine a biomarker for prostate cancer? J Sep Sci 34:3619–3621
Lad U, Khokhar S, Kale GM (2008) Electrochemical creatinine biosensors. Anal Chem 80:7910–7917
Li Q, Sritharathikhun P, Motomizu S, Motomizu S (2007) Development of novel reagent for Hantzsch reaction for the determination of formaldehyde by spectrophotometry and fluorometry. Anal Sci 23:413–417
Cavaliere B, Macchione B, Monteleone M, Naccarato A, Sindona G, Tagarelli A (2011) Sarcosine as a marker in prostate cancer progression: a rapid and simple method for its quantification in human urine by solid-phase microextraction-gas chromatography-triple quadrupole mass spectrometry. Anal Bioanal Chem 400:2903–2912
Cernei N, Zitka O, Ryvolova M, Adam V, Masarik M, Hubalek J, Kizek R (2012) Spectrometric and electrochemical analysis of sarcosine as a potential prostate carcinoma marker. Int J Electrochem Sci 7:4286–4301
Biavardi E, Tudisco C, Maffei F, Motta A, Massera C, Condorelli GG, Dalcanale E (2012) Exclusive recognition of sarcosine in water and urine by a cavitand-functionalized silicon surface. P Natl Acad Sci USA. doi:10.1073/pnas.1112264109
Sakharov IY, Castillo LJ, Areza JC, Galaev IY (2000) Purification and stability of peroxidase of african oil palm Elaies guineensis. Bioseparation 9:125–132
García-Salas P, Morales-Soto A, Segura-Carretero A, Fernández-Gutierrez A (2010) Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 15:8813–8826
Pierpoint WS (1996) The extraction of enzymes from plant tissues rich in phenolic compounds. Methods Mol Biol 59:69–80
Arnao MB, Acosta M, del Río JA, Varón R, García-Cánovas F (1990) A kinetic study on the suicide inactivation of peroxidase by hydrogen peroxide. Biochim Biophys Acta 1041:43–47
Acknowledgements
This work was supported by Consejería de Educación (Ref.: SA129A07) and Instituto Tecnológico Agrario (Ref.: ITACYL/SA-06-00-0) de la Junta de Castilla y León (Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez Galende, P., Manzano Muñoz, T., G. Roig, M. et al. Use of crude extract of lentil plant (Lens culinaris Medikus) in peroxidase-based analyses: fast kinetic determination of hydrogen peroxide and sarcosine in urine. Anal Bioanal Chem 404, 2377–2385 (2012). https://doi.org/10.1007/s00216-012-6343-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6343-6