Abstract
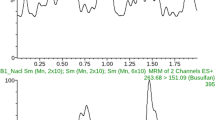
The role of busulfan (Bu) metabolites in the adverse events seen during hematopoietic stem cell transplantation and in drug interactions is not explored. Lack of availability of established analytical methods limits our understanding in this area. The present work describes a novel gas chromatography–tandem mass spectrometric assay for the analysis of sulfolane (Su) in plasma of patients receiving high-dose Bu. Su and Bu were extracted from a single 100 μL plasma sample by liquid–liquid extraction. Bu was separately derivatized with 2,3,5,6-tetrafluorothiophenolfluorinated agent. Mass spectrometric detection of the analytes was performed in the selected reaction monitoring mode on a triple quadrupole instrument after electronic impact ionization. Bu and Su were analyzed with separate chromatographic programs, lasting 5 min each. The assay for Su was found to be linear in the concentration range of 20–400 ng/mL. The method has satisfactory sensitivity (lower limit of quantification, 20 ng/mL) and precision (relative standard deviation less than 15 %) for all the concentrations tested with a good trueness (100 ± 5 %). This method was applied to measure Su from pediatric patients with samples collected 4 h after dose 1 (n = 46), before dose 7 (n = 56), and after dose 9 (n = 54) infusions of Bu. Su (mean ± SD) was detectable in plasma of patients 4 h after dose 1, and higher levels were observed after dose 9 (249.9 ± 123.4 ng/mL). This method may be used in clinical studies investigating the role of Su on adverse events and drug interactions associated with Bu therapy.

Overall sample preparation procedure for quantification of sulfolane and busulfan in plasma from patients receiving higher doses of busulfan



Similar content being viewed by others
References
Fry TJ (2010) Pediatr Blood Cancer 55:1043–1044
Schattenberg AV, Levenga TH (2006) Curr Opin Oncol 18:667–670
Krivoy N, Hoffer E, Lurie Y, Bentur Y, Rowe JM (2008) Curr Drug Saf 3:60–66
Bartelink IH, Bredius RG, Belitser SV, Suttorp MM, Bierings M, Knibbe CA, Egeler M, Lankester AC, Egberts AC, Zwaveling J, Boelens JJ (2009) Biol Blood Marrow Transplant 15:231–241
Zwaveling J, Bredius RG, Cremers SC, Ball LM, Lankester AC, Teepe-Twiss IM, Egeler RM, den Hartigh J, Vossen JM (2005) Bone Marrow Transplant 35:17–23
Czerwinski M, Gibbs JP, Slattery JT (1996) Drug Metab Dispos 24:1015–1019
Cooper AJ, Younis IR, Niatsetskaya ZV, Krasnikov BF, Pinto JT, Petros WP, Callery PS (2008) Drug Metab Dispos 36:1546–1552
Roberts JJ, Warwick GP (1961) Biochem Pharmacol 6:205–216
Marchand DH, bdel-Monem MM (1985) Biochem Biophys Res Commun 128:360–367
Hassan M, Oberg G, Ehrsson H, Ehrnebo M, Wallin I, Smedmyr B, Totterman T, Eksborg S, Simonsson B (1989) Eur J Clin Pharmacol 36:525–530
Ritter CA, Bohnenstengel F, Hofmann U, Kroemer HK, Sperker B (1999) J Chromatogr B: Biomed Sci Appl 730:25–31
Gibbs JP, Murray G, Risler L, Chien JY, Dev R, Slattery JT (1997) Cancer Res 57:5509–5516
Headley JV, Peru KM, Dickson LC (1999) J Chromatogr A 859:69–75
Ansari M, Lauzon-Joset JF, Vachon MF, Duval M, Theoret Y, Champagne MA, Krajinovic M (2010) Bone Marrow Transplant 45:261–267
Quernin MH, Poonkuzhali B, Montes C, Krishnamoorthy R, Dennison D, Srivastava A, Vilmer E, Chandy M, Jacqz-Aigrain E (1998) J Chromatogr B: Biomed Sci Appl 709:47–56
European Medicines Agency (EMEA). Draft guidelines on validation of bioanalytical methods (2012) http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. Accessed 6 Feb 2012.
Balasubramanian P, Srivastava A, Chandy M (2001) Clin Chem 47:766–768
Ehrsson H, Hassan M (1983) J Pharm Sci 72:1203–1205
Vassal G, Gouyette A, Hartmann O, Pico JL, Lemerle J (1989) Cancer Chemother Pharmacol 24:386–390
Embree L, Burns RB, Heggie JR, Phillips GL, Reece DE, Spinelli JJ, Hartley DO, Hudon NJ, Goldie JH (1993) Cancer Chemother Pharmacol 32:137–142
Stokvis E, Rosing H, Beijnen JH (2005) Mass Spectrom Rev 24:887–917
Ansari M, Uppugunduri CR, Deglon J, Theoret Y, Versace F, Gumy-Pause F, Ozsahin H, Dayer P, Desmules J, Daali Y (2012) Rapid Commun Mass Spectrom 26:1437–1446
Acknowledgments
We are thankful to the patients and their parents who consented to participate to this study. This study was supported by the grants of the CANSEARCH Foundation, The Hans Wilsdorf, the Télémaque Foundations, and the Geneva Cancer League.
Conflicts of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
François Versace and Chakradhara Rao S Uppugunduri contributed equally to this work.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(PDF 629 kb)
Rights and permissions
About this article
Cite this article
Versace, F., Uppugunduri, C.R.S., Krajinovic, M. et al. A novel method for quantification of sulfolane (a metabolite of busulfan) in plasma by gas chromatography–tandem mass spectrometry. Anal Bioanal Chem 404, 1831–1838 (2012). https://doi.org/10.1007/s00216-012-6330-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6330-y