Abstract
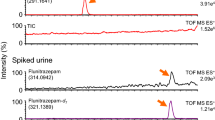
A simultaneous determination method based on ultra-performance liquid chromatography (UPLC) with fluorescence (FL) detection and electrospray-ionization time-of-flight mass spectrometry (ESI-TOF-MS) was developed for 16 “designated substances” (Shitei-Yakubutsu) controlled by the Pharmaceutical Affairs Law in Japan. These substances were first labeled with 4-(N,N-dimethylaminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole at 60 °C for 2 h in 0.1 M borax (pH 9.3). The resulting fluorophores were well separated by reversed-phase chromatography using an Acquity UPLC™ BEH C18 column (1.7 μm, 100 mm × 2.1 mm i.d.) by isocratic elution with a mixture of water and acetonitrile–methanol (20:80) containing 0.1% formic acid. The separated derivatives were sensitively detected by both FL and TOF-MS. However, the determination of several designated substances by FL detection showed interference from endogenous substances in biological samples. Therefore, the determination in real samples was carried out by a combination of UPLC separation and ESI-TOF-MS detection. The structures of the designated substances were identified from the protonated-molecular ions [M+H]+ obtained from the TOF-MS measurement. The calibration curves obtained from the peak area ratios of the internal standard (I.S.), i.e., 3-phenyl-1-propylamine, and the designated substances versus the injection amounts showed good linearity. The limits of detection \( \left( {{\text{S/N}} = 3} \right) \) and the limits of quantification \( \left( {{\text{S/N}} = 10} \right) \) in 0.1 mL of human plasma and urine for the present method were 0.30–150 pmol and 1.0–500 pmol, respectively. Good accuracy and precision (according to intraday and interday assays) were also obtained with the present procedure. This method was applied to analyses of human plasma, urine and real products.

Structures of designated substances tested






Similar content being viewed by others
References
Jacob III P, Shulgin AT (1994) Structure–activity relationships of the classic hallucinogens and their analogs. In: Lin GC, Glennon RA (eds) Hallucinogens: an update (NIDA Research Monograph 146). National Institute on Drug Abuse, Rockville, MD, pp. 74–91
Nicholos DE (1981) J Pharm Sci 70:839–849
Glennon RA, Rosecrans JA (1982) Neurosci Biobehav Rev 6:489–497
McKenna DJ, Towers GHN (1984) J Psychoact Drugs 16:347–358
Spoerke DG, Hall AH (1990) Emerg Med Clin North Am 8:579–593
Jacob P III, Shulgin AT (1994) NIDA Res Monogr 146:74–91
Marek GJ, Aghajanian GK (1998) Drug Alcohol Depend 51:189–198
Kanai K, Takekawa K, Kumamoto T, Ishikawa T, Ohmori T (2008) Forensic Toxicol 26:6–12
Buchanan JF, Brown CR (1988) Med Toxicol Adverse Drug Exp 3:1–17
Pichini S, Pujadas M, Marchei E, Pellegrini M, Fiz J, Pacifici R, Zuccaro P, Farre M, Torre R (2008) J Pharm Biomed Anal 47:335–342
Tancer ME, Johanson CE (2001) Drug Alcohol Depend 65:97–101
Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH (2006) Pharmacol Biochem Behav 83:122–129
Tanaka E, Kamata T, Katagi M, Tsuchihashi H, Honda K (2006) Forensic Sci Int 163:152–154
Alatrash G, Majhail NS, Pile JC (2006) Mayo Clin Proc 81:550–551
Wilson JM, McGeorge F, Smolinske S, Meatherall R (2005) Forensic Sci Int 148:31–36
Meatherall R, Sharma P (2003) J Anal Toxicol 27:313–317
Curtis B, Kemp P, Harty L, Choi C, Christensen D (2003) J Anal Toxicol 27:493–498
Klaassen T, Ho Pian KL, Westenberg HG, den Boer JA, van Praag HM (1998) Psychiatry Res 79:207–212
Smolinske S, Rastogi R, Schenkel RS (2003) J Toxicol Clin Toxicol 41:641
Balikova M (2005) Forensic Sci Int 153:85–91
Nakashima K (2006) Chemistry 61(12):12–16
Kikura-Hanajiri R, Kawamura M, Uchiyama N, Ogata J, Kamakura H, Saisho K, Goda Y (2008) Yakugaku Zasshi 128:971–979
Uchiyama N, Kawamura M, Kamakura H, Kikura-Hanajiri R, Goda Y (2008) Yakugaku Zasshi 128:981–987
Uchiyama N, Kikura-Hanajiri R, Kawahara N, Goda Y (2008) Yakugaku Zasshi 128:1499–1505
Doi K, Miyazawa M, Kojima T, Fujii H (2006) Yakugaku Zasshi 126:815–823
Frison G, Tedeschi L, Favretto D, Reheman A, Ferrara SD (2005) Rapid Commun Mass Spectrom 19:919–927
Staack RF, Maurer HH (2005) Curr Drug Metab 6:259–274
Matsumoto T, Kikura-Hanajiri R, Kamakura H, Kuwahara N, Goda Y (2005) J Health Sci 52:805–810
Chiu Y-C, Lin C-H, Chou S-H, Liu J-T (2004) J Chromatogr B 811:127–133
Theobald DS, Maurer HH (2004) J Chromatogr B 842:76–90
Theobald DS, Staack RF, Puetz M, Maurer HH (2005) J Mass Spectrom 40:1157–1172
Lin L-C, Lin C-H, Liu J-T, Chou S-H (2003) J Chromatogr B 798:241–247
Kikura-Hanajiri R, Hayashi M, Saisho K, Goda Y (2005) J Chromatogr B 825:29–37
Leung GNW, Leung DKK, Wan TSN, Wong CHF (2007) J Chromatogr A 1156:271–279
Gottardo R, Bortolotti F, De Paoil G, Pascali JP (2007) J Chromatogr A 1159:185–189
Elliott S, Wowe P, Symonds A (2004) Forensic Sci Int 139:183–190
Nakashima K (2005) J Health Sci 51:272–277
Macedo C, Branco PS, Ferreira LM, Lobo AM, Capela JP, Fernandes E, Bastos ML, Carvalho F (2007) J Health Sci 53:31–42
Nishida M, Yashiki M, Namera A, Kimura K (2006) J Chromatogr B 842:106–110
Jimenez C, De la Torre R, Ventura M, Segura J, Ventura R (2006) J Chromatogr B 843:84–93
Kumihashi M, Ameno K, Shibayama T, Suga K, Miyauchi H, Jamal M, Wang W, Uekita I, Ijiri I (2007) J Chromatogr B 845:180–183
Carrera V, Sabater E, Vilanova E, Sogorb MA (2007) J Chromatogr B 847:88–94
Trachsel D (2003) Helv Chim Acta 86:2754–2759
Appollonio LG, Whittall IR, Pianca DJ, Kyd JM, Maher WA (2006) Rapid Commun Mass Spectrom 20:2259–2264
Appollonio LG, Pianca DJ, Whittall IR, Maher WA, Kyd JM (2006) J Chromatogr B 836:111–115
Min JZ, Shimizu Y, Toyo’oka T, Inagaki S, Kikura-Hanajiri R, Goda Y (2008) J Chromatogr B 873:187–194
Nakamura S, Wada M, Crabtree BL, Reeves PM, Montgomery JH, Byrd HJ, Harada S, Kuroda N, Nakashima K (2007) Anal Bioanal Chem 387:1983–1990
Wu N, Collins DC, Lippert JA, Xiang Y, Lee ML (2000) J Microcol Sep 12:462–469
Wu Y-H, Lin K-L, Chen S-C, Chang Y-Z (2008) Rapid Commun Mass Spectrom 22:887–897
Acknowledgment
The present research was supported in part by a Health Sciences Research Grant from the Ministry of Health Labor and Welfare in Japan, and a Research Grant from the Ministry of Education, Science, Sports and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Min, J.Z., Hatanaka, S., Toyo’oka, T. et al. Rapid, sensitive and simultaneous determination of fluorescence-labeled designated substances controlled by the Pharmaceutical Affairs Law in Japan by ultra-performance liquid chromatography coupled with electrospray-ionization time-of-flight mass spectrometry. Anal Bioanal Chem 395, 1411–1422 (2009). https://doi.org/10.1007/s00216-009-3046-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3046-8