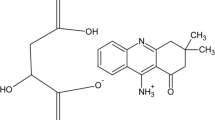
Abstract
The epidermal growth factor receptor (EGFR) plays a key role in the pathogenesis of cancers of different types. It has been shown that EGFR and EGF-like peptides are often overexpressed in human carcinomas and that these proteins can cause cell transformation both in vivo and in vitro. In order to design a new apoptotic EGFR inhibitor, we used the essential pharmacophoric structural properties of EGFR inhibitors. We started with the natural alkaloid, theobromine, to get a new semisynthetic N-cyclohexyl acetamide derivative (T-1-NCA). T-1-NCA was extensively examined computationally for its potential against the EGFR protein. We initially performed deep density functional theory (DFT) computations to validate its 3D structure. The electrostatic potential, global reactive indices, and total density of states anticipating a high degree of reactivity were also indicated by the DFT analyses. Second, T-1-NCA's propensity to bind and inhibit the EGFR protein was investigated and verified using structure-based computational investigations such as molecular docking against EGFRWT, molecular dynamics (MD) over 100 ns, MM-GPSA, and PLIP experiments. T-1-NCA's computational ADME and toxicity profiles were examined before the synthesis, and its safety and general drug-likeness were anticipated. As a consequence, T-1-NCA was semi-synthesized to examine the proposed design and the in silico findings. In comparison with erlotinib, T-1-NCA suppressed EGFRWT in vitro with an IC50 value of 24.25 nM. (5.87 nM). Furthermore, T-1-NCA suppressed the proliferation of A549 and HCT-116 malignant cell lines with IC50 values of 40.20 and 34.05 µM, respectively, as compared to erlotinib, which had IC50 values of 17.13 and 17.32 µM. Interestingly, T-1-NCA’s selectivity indices were 3.29 and 3.89 against the two cancer cell lines indicating its general safety. Finally, the apoptotic effects of T-1-NCA were confirmed by flow cytometry and RT-PCR through the significant increase of the levels BAX, Casp3, and Casp9 in addition to the significant decrease of Bcl-2 level.

















Similar content being viewed by others
Data availability
Data are available with corresponding authors upon request. Sample availability: T-1-NCA is available from the authors.
References
Nandi S, Bagchi MC (2022) Exploring CDKs, Ras-ERK, and PI3K-Akt in abnormal signaling and cancer. J Cancer Res Updates 11:63–69
Siegel RL et al (2022) Cancer statistics 72(1): 7–33
Khan F, Akhtar S, Kamal MA (2023) Nanoinformatics and personalized medicine: an advanced cumulative approach for cancer management. Curr Med Chem 30(3):271–285
Voss AK, Strasser A (2020) The essentials of developmental apoptosis. F1000Research, 9
Carneiro BA, El-Deiry WS (2020) Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol 17(7):395–417
Goel S, Hidalgo M, Perez-Soler R (2007) EGFR inhibitor-mediated apoptosis in solid tumors. J Experiment Therapeutics Oncol 6(4)
Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, Pao W (2007) Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med 4(10):e294
Nandi S et al (2022) Natural Sourced inhibitors of EGFR, PDGFR, FGFR and VEGFRMediated signaling pathways as potential anticancer agents. Curr Med Chem 29(2):212–234
Rosenkranz AA, Slastnikova TA (2020) Epidermal growth factor receptor: key to selective intracellular delivery. Biochemistry (Mosc) 85(9):967–1092
Kaufman NEM et al (2021) Molecular targeting of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR). Molecules 26(4):1076
Normanno N et al (2001) The role of EGF-related peptides in tumor growth. Front Biosci 6(3):D685-707
Spano JP et al (2005) Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol 16(1):102–108
Tripathi SK et al (2020) Recent updates on the resistance mechanisms to epidermal growth factor receptor tyrosine kinase inhibitors and resistance reversion strategies in lung cancer. Med Res Rev 40(6):2132–2176
Nandi S, Bagchi MC (2011) In silico design of potent EGFR kinase inhibitors using combinatorial libraries. Mol Simul 37(03):196–209
Durgapal J et al (2018) QSAR and structure-based docking studies of aryl pyrido [2, 3-d] pyrimidin-7 (8H)-ones: an attempt to anticancer drug design. Int J Quantitat Struct-Property Relationships (IJQSPR) 3(1):43–73
da Rosa R, Schenkel EP, Campos Bernardes LS (2020) Semisynthetic and newly designed derivatives based on natural chemical scaffolds: moving beyond natural products to fight Trypanosoma cruzi. Phytochem Rev 19:105–122
Tan S, Lu R, Yao D, Wang J, Gao P, Xie G, Yao X (2023) Identification of LRRK2 inhibitors through computational drug repurposing. ACS Chem Neurosci 14(3):481–493
Ligand-based S-b (2023) Computer-aided drug design. Curr Drug Synth p 339
Lin X, Li X, Lin X (2020) A review on applications of computational methods in drug screening and design. Molecules 25(6):1375
Bertaccini EJ (2023) Anesthesia, coming of age in the world of modern in silico drug design. Anesthesiology 138(2):129–131
Cohen NC (ed) (1996) Guidebook on molecular modeling in drug design. Gulf Professional Publishing
Keith JA et al (2021) Combining machine learning and computational chemistry for predictive insights into chemical systems. Chem Rev 121(16):9816–9872
Metwaly AM et al (2023) Preparation and characterization of patuletin-loaded chitosan nanoparticles with improved selectivity and safety profiles for anticancer applications. J Chem 2023:6684015
Moroy G, Martiny VY, Vayer P, Villoutreix BO, Miteva MA (2012) Toward in silico structure-based ADMET prediction in drug discovery. Drug Discov Today 17(1–2):44–55
Metwaly AM, Elkaeed EB, Alsfouk BA, Saleh AM, Mostafa AE, Eissa IH (2022) The computational preventive potential of the rare flavonoid, patuletin, isolated from tagetes patula, against SARS-CoV-2. Plants 11(14):1886
Tielens F et al (2020) Characterization of amorphous silica based catalysts using DFT computational methods. Catal Today 354:3–18
Pracht P, Bohle F, Grimme S (2020) Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys Chem Chem Phys 22(14):7169–7192
Eissa IH et al (2023) A theobromine derivative with anticancer properties targeting VEGFR-2: semisynthesis, in silico and in vitro studies. ChemistryOpen 12(10):e202300066
Chalkha M et al (2022) Crystallographic study, biological assessment and POM/Docking studies of pyrazoles-sulfonamide hybrids (PSH): identification of a combined antibacterial/antiviral pharmacophore sites leading to in-silico screening the anti-Covid-19 activity. J Mol Struct 1267:133605
Eissa IH, Yousef RG, Elkady H, Alsfouk AA, Alsfouk BA, Husein DZ, Metwaly AM (2023) A new anticancer semisynthetic theobromine derivative targeting EGFR protein: CADDD study. Life 13(1):191
Elkaeed EB, Yousef RG, Elkady H, Alsfouk AA, Husein DZ, Ibrahim IM, Eissa IH (2022) A new theobromine-based EGFRWT and EGFRT790M inhibitor and apoptosis inducer: design, semi-synthesis, docking, DFT, MD simulations, and in vitro studies. Processes 10(11):2290
Elkaeed EB, Yousef RG, Elkady H, Alsfouk AA, Husein DZ, Ibrahim IM, Eissa IH (2022) New anticancer theobromine derivative targeting egfrwt and egfrt790m: design, semi-synthesis, in silico, and in vitro anticancer studies. Molecules 27(18):5859
Sobh EA et al (2023) Design, synthesis, docking, MD simulations, and anti-proliferative evaluation of thieno[2,3-d]pyrimidine derivatives as new EGFR inhibitors. J Enzyme Inhib Med Chem 38(1):2220579
Sobh EA, Dahab MA, Elkaeed EB, Alsfouk AA, Ibrahim IM, Metwaly AM, Eissa IH (2023) Discovery of new thieno [2, 3-d] pyrimidines as EGFR tyrosine kinase inhibitors for cancer treatment. Future Med Chem 15(13):1167–1184
Sobh EA, Dahab MA, Elkaeed EB, Alsfouk AA et al. (2023). Computer aided drug discovery (CADD) of a thieno [2, 3-d] pyrimidine derivative as a new EGFR inhibitor targeting the ribose pocket. J Biomol Struct Dynam pp 1–23
Eissa IH, Yousef RG, Elkady H, Elkaeed EB, Husein DZ, Ibrahim IM, Metwaly AM (2023) New theobromine derivative as apoptotic anti-triple-negative breast cancer targeting EGFR protein: CADD story. J Mol Struct 1294:136336
Eissa IH, Yousef GR, Elkady H, Alsfouk AA et al. (2023) New apoptotic anti-triple-negative breast cancer theobromine derivative inhibiting EGFRWT and EGFRT790M: in silico and in vitro evaluation. Mol Divers, pp 1-21
Eissa IH et al (2023) Anticancer derivative of the natural alkaloid, theobromine, inhibiting EGFR protein: computer-aided drug discovery approach. PLoS ONE 18(3):e0282586
Lee HYJ et al (2021) Medicinal herbs and bioactive compounds overcome the drug resistance to epidermal growth factor receptor inhibitors in non-small cell lung cancer. Oncol Lett 22(3):1–10
Salehi B et al (2018) Resveratrol: a double-edged sword in health benefits. Biomedicines 6(3):91
Zhu Y et al (2015) Resveratrol overcomes gefitinib resistance by increasing the intracellular gefitinib concentration and triggering apoptosis, autophagy and senescence in PC9/G NSCLC cells. Sci Rep 5(1):1–12
Lee J-Y et al (2011) Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: the versatile adjuvant for gefitinib therapy. PLoS ONE 6(8):e23756
Chen P et al (2019) Curcumin overcome primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death. J Exp Clin Cancer Res 38(1):1–17
Li X et al (2017) Shikonin inhibits gefitinib-resistant non-small cell lung cancer by inhibiting TrxR and activating the EGFR proteasomal degradation pathway. Pharmacol Res 115:45–55
Xu L et al (2018) Gambogenic acid inhibits fibroblast growth factor receptor signaling pathway in erlotinib-resistant non-small-cell lung cancer and suppresses patient-derived xenograft growth. Cell Death Dis 9(3):1–14
Wang Z et al (2016) Cordycepin induces apoptosis and inhibits proliferation of human lung cancer cell line H1975 via inhibiting the phosphorylation of EGFR. Molecules 21(10):1267
Hawley SA, Ross FA, Russell FM, Atrih A, Lamont DJ, Hardie DG (2020) Mechanism of activation of AMPK by Cordycepin. Cell Chem Biol 27(2):214–222
Wang Z et al (2010) Binding of Cordycepin monophosphate to AMP-activated protein kinase and its effect on AMP-activated protein kinase activation. Chem Biol Drug Des 76(4):340–344
Wei C et al (2019) Cordycepin inhibits drug-resistance non-small cell lung cancer progression by activating AMPK signaling pathway. Pharmacol Res 144:79–89
Nasser AA et al (2020) Discovery of new pyrimidine-5-carbonitrile derivatives as anticancer agents targeting EGFR WT and EGFR T790M. Org Biomol Chem 18(38):7608–7634
Elkaeed EB et al (2022) New anticancer theobromine derivative targeting egfrwt and egfrt790m: design, semi-synthesis, in silico, and in vitro anticancer studies. Molecules 27(18):5859
Husein DZ, Hassanien R, Khamis M (2021) Cadmium oxide nanoparticles/graphene composite: synthesis, theoretical insights into reactivity and adsorption study. RSC Adv 11(43):27027–27041
Nandi S et al (2013) Quantitative structure-activation barrier relationship modeling for Diels-Alder ligations utilizing quantum chemical structural descriptors. Chem Cent J 7(1):1–13
Wang T, Husein DZ (2022) Novel synthesis of multicomponent porous nano-hybrid composite, theoretical investigation using DFT and dye adsorption applications: disposing of waste with waste. Environ Sci Pollut Res 30(4):8928–8955
Nandi S, Bagchi MC (2010) 3D-QSAR and molecular docking studies of 4-anilinoquinazoline derivatives: a rational approach to anticancer drug design. Mol Diversity 14:27–38
Nossier ES, Alasfoury RA, Hagras M, El-Manawaty M, Sayed SM, Ibrahim IM, Elzahabi HS (2022) Modified pyrido [2, 3-d] pyrimidin-4 (3H)-one derivatives as EGFRWT and EGFRT790M inhibitors: design, synthesis, and anti-cancer evaluation. J Mol Struct 1270:133971
Lipinski CA et al (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23(1–3):3–25
Chuang KV, Gunsalus LM, Keiser MJ (2020) Learning molecular representations for medicinal chemistry: miniperspective. J Med Chem 63(16):8705–8722
Ferreira LLG, Andricopulo AD (2019) ADMET modeling approaches in drug discovery. Drug Discov Today 24(5):1157–1165
Idakwo G et al (2018) A review on machine learning methods for in silico toxicity prediction. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 36(4):169–191
Kruhlak NL et al (2012) (Q)SAR modeling and safety assessment in regulatory review. Clin Pharmacol Ther 91(3):529–534
Obeng E (2020) Apoptosis (programmed cell death) and its signals-A review. Braz J Biol 81:1133–1143
Wyllie AH (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284:555–556
Taghour MS et al (2022) Benzoxazole derivatives as new VEGFR-2 inhibitors and apoptosis inducers: design, synthesis, in silico studies, and antiproliferative evaluation. J Enzyme Inhib Med Chem 37(1):2063–2077
Elwan A et al (2022) Modified benzoxazole-based VEGFR-2 inhibitors and apoptosis inducers: design, synthesis, and anti-proliferative evaluation. Molecules 27(15):5047
Suleimen YM, Jose RA, Mamytbekova GK, Suleimen RN, Ishmuratova MY, Dehaen W, Metwaly AM (2022) Isolation and in silico inhibitory potential against SARS-CoV-2 RNA polymerase of the rare Kaempferol 3-O-(6 ″-O-acetyl)-Glucoside from Calligonum tetrapterum. Plants 11(15):2072
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1:19–25
Brooks BR, Brooks CL III, Mackerell AD Jr, Nilsson L, Petrella RJ, Roux B, Karplus M (2009) CHARMM: the biomolecular simulation program. J Comput Chem 30(10):1545–1614
Jo S, Cheng X, Islam SM, Huang L, Rui H, Zhu A, Im W (2014) CHARMM-GUI PDB manipulator for advanced modeling and simulations of proteins containing nonstandard residues. Advances Protein Chem Struct Biol 96:235–265
Tuccinardi T (2021) What is the current value of MM/PBSA and MM/GBSA methods in drug discovery? Expert Opin Drug Discov 16(11):1233–1237
Valdés-Tresanco MS, Valdés-Tresanco ME, Valiente PA, Moreno E (2021) gmx_MMPBSA: a new tool to perform end-state free energy calculations with GROMACS. J Chem Theory Comput 17(10):6281–6291
Amadei A, Linssen AB, Berendsen HJ (1993) Essential dynamics of proteins. Proteins Struct, Funct Bioinf 17(4):412–425
Papaleo E, Mereghetti P, Fantucci P, Grandori R, De Gioia L (2009) Free-energy landscape, principal component analysis, and structural clustering to identify representative conformations from molecular dynamics simulations: the myoglobin case. J Mol Graph Modell 27(8):889–899
Biovia DS (2017) Discovery studio modeling environment, Release
Yousef RG et al (2022) (E)-N-(3-(1-(2-(4-(2, 2, 2-Trifluoroacetamido) benzoyl) hydrazono) ethyl) phenyl) nicotinamide: a novel pyridine derivative for inhibiting vascular endothelial growth factor receptor-2: synthesis, computational, and anticancer studies. Molecules 27(22):7719
Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Boyd MR (1988) Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 48(3):589–601
Van de Loosdrecht AA, Beelen RHJ, Ossenkoppele G, Broekhoven MG, Langenhuijsen MMAC (1994) A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods 174(1–2):311–320
Koch A et al (2005) Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J Ethnopharmacol 101(1–3):95–99
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2023R116), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Research Center at AlMaarefa University for funding this work.
Author information
Authors and Affiliations
Contributions
A.M, I.E and E.E Planned the work, A.M and I.E supervised the expemints, R.Y, M.A., and H.E made the synthesis and molecular docking, DH made the DFT, I.I made the MD simulations, A.A, E.E, participated in writing, revision and Funding. All authors revised and approved the final version of manuscript
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest to be declared.
Institutional review board statement
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eissa, I.H., G.Yousef, R., Elkady, H. et al. A new anticancer derivative of the natural alkaloid, theobromine, as an EGFR inhibitor and apoptosis inducer. Theor Chem Acc 143, 1 (2024). https://doi.org/10.1007/s00214-023-03071-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03071-z