Abstract
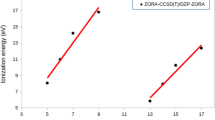
Employing H-like spin-orbitals (SOs) in electronic structure theory is a long-awaited quantum problem as the analytical integral of Coulomb interaction is very difficult to solve for one-center many-electron (1c-ne) system. He-isoelectronic ions become a benchmark. Complexity grows fast for Period-II s- and p-block elements with increasing number of electrons. Moreover, Hartree-Fock Self-Consistent Field (SCF) and post Hartree-Fock SCF theories generally make use of closed-shell, restricted and unrestricted open-shell single configurations (SCs) but actual electronic bound states urge for multiconfigurations (MCs). After Born-Oppenheimer (BO) approximation, utilization of associated Laguerre polynomial/Whittaker-M function basis sets of H-like SOs for the Coulomb Green\('\)s function among electrons furnishes analytical, terminable, simple and finitely summed integrals in terms of Lauricella functions. MCs complying with so-called ground, singly and multiply excited states incurring s- and p-SOs are constructed to capture monopole and dipole factors only. However, we believe that quadrupole and higher order poles can be achieved as a product of angular integrals using Wigner 3-j symbols and closed forms of radial integrals. We have observed good agreement among literature and exact ground state energies (GSEs) of He-isoelectronic ions and Period-II elements with both their so-called ground electronic configurations as well as MCs. For certain elements, we have found satisfactory results for ionization energies (IEs).



Similar content being viewed by others
References
Kellner GW (1927) Zeitschrift für Physik 44:91. https://doi.org/10.1007/BF01391720
Hylleraas EA (1929) E.A. Zeitschrift für Physik 54:347. https://doi.org/10.1007/BF01375457
Hylleraas EA (1930) Zeitschrift für Physik 65:209. https://doi.org/10.1007/BF01397032
Kinoshita T (1957) Phys Rev 105:1490. https://doi.org/10.1103/PhysRev.105.1490
Pekeris CL (1959) Phys Rev 115:1216. https://doi.org/10.1103/PhysRev.115.1216
Frankowski K, Pekeris CL (1966) Phys Rev 146:46. https://doi.org/10.1103/PhysRev.146.46
Bhattacharyya S, Bhattacharyya A, Talukdar B, Deb NC (1996) J Phys B: Atomic, Molecular Optical Phys 29:L147. https://doi.org/10.1088/0953-4075/29/5/003
Korobov VI (2002) Phys Rev A 66:024501. https://doi.org/10.1103/PhysRevA.66.024501
Roothaan CCJ (1960) Rev Mod Phys 32:179. https://doi.org/10.1103/RevModPhys.32.179
Sahni V, Krieger JB (1971) Int J Quantum Chem 5:47. https://doi.org/10.1002/qua.560050807
Combescot R (2017) Phys Rev X 7:041035. https://doi.org/10.1103/PhysRevX.7.041035
Fock V (1954) Izv Akad Nauk SSSR Ser Fiz 18:161
Scherr CW (1960) J Chem Phys 33:317. https://doi.org/10.1063/1.1731129
Scherr CW, Knight RE (1963) Rev Mod Phys 35:436. https://doi.org/10.1103/RevModPhys.35.436
Nakashima H, Nakatsuji H (2007) J Chem Phys 127:224104. https://doi.org/10.1063/1.2801981
Kato T (1957) Commun Pure Appl Math 10:151. https://doi.org/10.1002/cpa.3160100201
Sharma S, Aggarwal P, Kaur H, Hazra RK (2019) J Indian Chem Soc 96:775
Sharma S, Aggarwal P, Hazra RK (2020) Mol Phys 118:e1770881. https://doi.org/10.1080/00268976.2020.1770881
Sharma S, Kapil B, Aggarwal P, Hazra RK (2022) Physics Open 11:100107. https://doi.org/10.1016/j.physo.2022.100107
Kapil B, Sharma S, Aggarwal P, Hazra RK (2022) Eur Phys J Plus 137:809. https://doi.org/10.1140/epjp/s13360-022-02970-7
Poszwa A (2020) Physica E 124:114247. https://doi.org/10.1016/j.physe.2020.114247
Clementi E, Veillard A (1966) J Chem Phys 44:3050. https://doi.org/10.1063/1.1727179
Clementi E, Roothaan CCJ, Yoshimine M (1962) Phys Rev 127:1618. https://doi.org/10.1103/PhysRev.127.1618
Clementi E (1963a) Correlation Energy for Atomic Systems. J Chem Phys 38:2248. https://doi.org/10.1063/1.1733957
Clementi E (1963b) J Chem Phys 38:1001. https://doi.org/10.1063/1.1733745
Schmidt KE, Moskowitz JW (1990) J Chem Phys 93:4172. https://doi.org/10.1063/1.458750
Moskowitz JW, Schmidt KE (1992) J Chem Phys 97:3382. https://doi.org/10.1063/1.463938
Alexander SA, Coldwell RL (1995) J Chem Phys 103:2572. https://doi.org/10.1063/1.469679
Lin X, Zhang H, Rappe AM (2000) J Chem Phys 112:2650. https://doi.org/10.1063/1.480839
Browne JC, Miller J (1962) J Chem Phys 36:2324. https://doi.org/10.1063/1.1732884
Shull H (1959) J Chem Phys 30:1405. https://doi.org/10.1063/1.1730212
Hagstrom S, Shull H (1959) J Chem Phys 30:1314. https://doi.org/10.1063/1.1730179
Jones WD, Brooks FL (1960) J Chem Phys 32:124. https://doi.org/10.1063/1.1700884
Levine IN (2014) Quantum chemistry, 7th edn. Pearson, Boston
Dutta J, Mukherjee S, Naskar K, Ghosh S, Mukherjee B, Ravi S, Adhikari S (2020) Phys Chem Chem Phys 22:27496. https://doi.org/10.1039/D0CP04052E
Baer M (2002) Phys Rep 358:75. https://doi.org/10.1016/S0370-1573(01)00052-7
Mukherjee B, Naskar K, Mukherjee S, Ghosh S, Sahoo T, Adhikari S (2019) Int Rev Phys Chem 38:287. https://doi.org/10.1080/0144235X.2019.1672987
Feynman RP (1939) Phys Rev 56:340. https://doi.org/10.1103/PhysRev.56.340
Lichten W (1967) Phys Rev 164:131. https://doi.org/10.1103/PhysRev.164.131
Smith FT (1969) Phys Rev 179:111. https://doi.org/10.1103/PhysRev.179.111
Arfken G, Weber H (2005) Mathematical Methods for Physicists. Elsevier, Netherlands
Pilar FL (1968) Elementary quantum chemistry, Quantum Mechanics. McGraw-Hill, New York
Merzbacher E (1998) Quantum Mechanics. Wiley, New York
Cohen-Tannoudji C, Diu B, Laloë F (1977) Quantum Mechanics. Wiley, New York
Griffiths D (1999) Introduction to Electrodynamics. Prentice Hall, Hoboken
Jackson J (1999) Classical Electrodynamics. John Wiley & Sons, Hoboken
Messiah A (1999) Quantum Mechanics, Dover books on physics. Dover Publications, Mineola
Friedrich H (1991) Theoretical atomic physics. Springer-Verlag, Berlin, p 115
Kramida A, Ralchenko Yu, Reader J (2021) and NIST ASD Team, howpublished NIST Atomic Spectra Database (ver. 5.9), [Online] National Institute of Standards and Technology, Gaithersburg, MD
Sarsa A, Gálvez FJ, Buendía E (1998) J Chem Phys 109:3346. https://doi.org/10.1063/1.476929
Eyring H, Walter J, Kimball G (1961) Quantum Chemistry. John Wiley & Sons, Incorporated, New York
Antonsen F (1999) Phys Rev A 60:812. https://doi.org/10.1103/physreva.60.812
Drake GW (1988) Can J Phys 66:586. https://doi.org/10.1139/p88-100
Yerokhin VA, Pachucki K (2010) Phys Rev A 81:022507. https://doi.org/10.1103/PhysRevA.81.022507
Erdélyi A (1937) Monatshefte für Mathematik und Physik 46:1. https://doi.org/10.1007/BF01792661
Acknowledgements
We express our sincere thanks to Professor Shankar Prasad Bhattacharyya for his continuous motivation. Our sincere thanks go to CSIR (SRF scheme) and FRP Grant under Institution of Eminence, University of Delhi (Ref. No./IoE/2021/12/FRP) for their financial support.
Author information
Authors and Affiliations
Contributions
BK and RKH wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A Standard equations and integrals
Associated Laguerre Polynomial [17,18,19,20, 41]
Lower and Upper Incomplete Gamma function [17,18,19,20, 41]
Standard Integral-I (Erd\(\acute{e}\)lyi’s Integral) [17,18,19,20, 55]
Appendix B Degeneracies of lowest bound states
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kapil, B., Sharma, S., Aggarwal, P. et al. Analytical multiconfiguration treatment to one-center many-electron He-isoelectronic ions and Period-II elements with H-like bound-states. Theor Chem Acc 142, 90 (2023). https://doi.org/10.1007/s00214-023-03011-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03011-x