Abstract
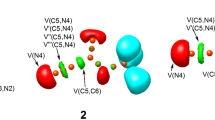
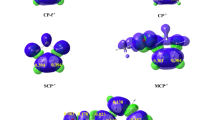
The [3+2] Cycloaddition (32CA) reaction of nitrile ylide (NY) 10 with electron-deficient ethylene 11 has been studied within the molecular electron density theory through DFT calculations at the MPWB1K/6-31G(d) computational level. A structural analysis of NY 10 indicates that this three-atom component has a carbenoid structure, allowing its participation in carbenoid-type (cb-type) 32CA reactions. This 32CA reaction takes place through a one-step mechanism with very low activation energy, 2.3 kcal mol−1. In gas phase, this 32CA reaction is not stereoselective and has low regioselectivity. Inclusion of solvent effects does not modify the activation energy, but increases the meta regioselectivity in clear agreement with the experimental outcomes. Electron localisation function topological analysis for the formation of the two C–C single bonds along the four competitive channels associated with this 32CA reaction makes it possible to characterise two dissimilar mechanisms. Along the more favourable meta regioisomeric channels, the 32CA reaction takes place through a two-stage one-step mechanism, while along the ortho regioisomeric channels it takes place via a synchronous C–C bond formation process.











Similar content being viewed by others
References
Huisgen R (1984) In: Padwa A (ed) 1,3-dipolar cycloaddition chemistry, vol 1. Wiley, New York
Carruthers W (1990) In: Baldwin JE, Magnus PD (eds) Cycloaddition reactions in organic synthesis. Pergamon, Oxford
Padwa A, Pearson WH (eds) (2002) Synthetic applications of 1,3-dipolar Cycloaddition chemistry toward heterocycles and natural products, vol 59. Wiley, New York
Bailly C (2004) Curr Med Chem-AntiCancer Agents 4:364–378
Bellina F, Rossi R (2006) Tetrahedron 62:7213–7256
Domingo LR, Emamian SR (2014) Tetrahedron 70:1267–1273
Gothelf KV, Jorgensen KA (1998) Chem Rev 98:863–910
Domingo LR, Sáez JA (2009) Org Biomol Chem 7:3576–3583
Ess DH, Houk KN (2008) J Am Chem Soc 130:10187–10198
Ess DH, Houk KN (2008) J Am Chem Soc 129:10646–10647
Osuna S, Houk KN (2009) Chem Eur J 15:13219–13231
Bickelhaupt FM (1999) J Comput Chem 20:114–128
Fernández I, Bickelhaupt FM (2014) Chem Soc Rev 43:4953–4967
Hammond GS (1955) J Am Chem Soc 77:334–338
Hohenberg P, Kohn W (1964) Phys Rev B 136:864–871
Domingo LR (2014) RSC Adv 4:32415–32428
Ríos-Gutiérrez M, Domingo LR, Pérez P (2015) RSC Adv 5:84797–84809
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Tetrahedron 72:1524–1532
Domingo LR, Chamorro E, Pérez P (2010) Lett Org Chem 7:432–439
Sustmann R (1974) Pure Appl Chem 40:569–593
Fukui K (1964) In: Löwdin PO, Pullman B (eds) Molecular orbitals in chemistry physics and biology. Academic Press, New York
Krokidis X, Noury S, Silvi B (1997) J Phys Chem A 101:7277–7282
Huisgen R, Stangl H, Sturm HJ, Wagenhofer H (1962) Angew Chem 74:31
Bunge K, Huisgen R, Raab R, Stangl H (1972) Chem Ber 105:1279–1295
Sibi MP, Soeta T, Jasperse CP (2009) Org Lett 11:5366–5369
Zhao Y, Truhlar DG (2004) J Phys Chem A 108:6908–6918
Hehre WJ, Radom L, PvR Schleyer, Pople J (1986) Ab initio molecular orbital theory. Wiley, New York
Schlegel HB (1982) J Comput Chem 2:214–218
Schlegel HB (1994) In: Yarkony DR (ed) Modern electronic structure theory. World Scientific Publishing, Singapore
Fukui K (1970) J Phys Chem 74:4161–4163
González C, Schlegel HB (1990) J Phys Chem 94:5523–5527
González C, Schlegel HB (1991) J Chem Phys 95:5853–5860
Tomasi J (1994) Persico M 94:2027–2094
Simkin BY, Sheikhet I (1995) Quantum chemical and statistical theory of solutions—computational approach. Ellis Horwood, London
Cances E, Mennucci B, Tomasi J (1997) J Chem Phys 107:3032–3041
Cossi M, Barone V, Cammi R, Tomasi J (1996) Chem Phys Lett 255:327–335
Barone V, Cossi M, Tomasi J (1998) J Comput Chem 19:404–417
Reed AE, Weinstock RB, Weinhold FJ (1985) J Chem Phys 83:735–746
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Parr RG, von Szentpaly L, Liu S (1999) J Am Chem Soc 121:1922–1924
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512–7514
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Domingo LR, Chamorro E, Pérez P (2008) J Org Chem 73:4615–4624
Domingo LR, Pérez P (2011) Org Biomol Chem 9:7168–7175
Kohn W, Sham L (1965) J Phys Rev 140:1133–1138
Domingo LR, Pérez P, Sáez JA (2013) RSC Adv 3:1486–1494
Noury S, Krokidis K, Fuster F, Silvi B (1999) Comput Chem 23:597–604
Frisch MJ et al (2009) Gaussian 09, Revision A.02. Gaussian Inc, Wallingford CT
Geerlings P, De Proft F, Langenaeker W (2003) Chem Rev 103:1793–1873
Ess DH, Jones GO, Houk KN (2006) Adv Synth Catal 348:2337–2361
Fernández-Herrera MA, Zavala-Oseguera C, Cabellos JL, Sandoval-Ramírez J, Domingo LR, Merino G (2014) J Mol Model 20:2207
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Tetrahedron 58:4417–4423
Jaramillo P, Domingo LR, Chamorro E, Pérez P (2008) J Mol Struct (Theochem) 865:68–72
Benchouk W, Mekelleche SM, Silvi B, Aurell MJ, Domingo LR (2011) J Phys Org Chem 24:611–618
Acknowledgments
This work is in honour of the 60th birthday of Professor Alberto Vela. This work has been supported by the Ministry of Economy and Competitiveness of the Spanish Government, project CTQ2013-45646-P, Fondecyt (Chile) grants 1140341 (P.P.), 1140343 (E.C.) and 11130589 (M.D.-N), Millennium Nucleus Chemical Processes and Catalysis (CPC) project No. 120082 and the Universidad Andrés Bello (UNAB) for continuous support through research grants DI-793-15/R and DI-806-15/R. Prof L.R.D. also thanks FONDECYT for continuous support through Cooperación Internacional. M. R.-G. thanks the Ministry of Economy and Competitiveness for a pre-doctoral contract co-financed by the European Social Fund (BES-2014-068258).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published as part of the special collection of articles “Festschrift in honour of A. Vela”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Domingo, L.R., Ríos-Gutiérrez, M., Duque-Noreña, M. et al. Understanding the carbenoid-type reactivity of nitrile ylides in [3+2] cycloaddition reactions towards electron-deficient ethylenes: a molecular electron density theory study. Theor Chem Acc 135, 160 (2016). https://doi.org/10.1007/s00214-016-1909-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1909-6