Abstract
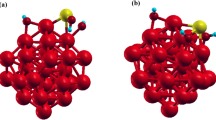
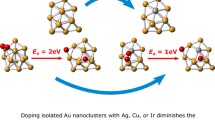
The adsorption of trivalent arsenic [As(OH)3] onto the bimetallic Au19Cu and Au19Pd clusters was computationally studied to get insights about as embedded electrodes with bimetallic gold nanostructures can serve for the adsorption and subsequent sensing of arsenic traces from polluted waters. It was found that the As(OH)3 chemisorption onto Au19Cu and Au19Pd is reached with adsorption energies of up to 1.02 and 1.48 eV, respectively, with even a stable physisorption with energies of up to 0.57 eV. Both arsenic and oxygen atoms were determined to bind with adsorbent Cu and Pd atoms, and all the interactions appear to be stable in an aqueous environment. The stability of the adsorbent–pollutant interactions was explained in terms of metal–pollutant chemical binding, charge transfer, dispersion force interactions and non-conventional OH···Au hydrogen bonds. Moreover, the As(OH)3 adsorption on the metal clusters is also accompanied by changes in the reactivity, electron transfer and decreases of the HOMO–LUMO energy gap with respect to the isolated substrates; the highest changes obtained in the mentioned properties are classified in the order Au19Pd > Au19Cu > Au20.








Similar content being viewed by others
References
Mondal P, Majumder C, Mohanty B (2006) Laboratory based approaches for arsenic remediation from contaminated water: recent developments. J Hazard Mater 137(1):464–479
Bundschuh J, Bhattacharya P, Sracek O, Mellano MF, Ramírez AE, Storniolo AdR, Martín RA, Cortés J, Litter MI, Jean J-S (2011) Arsenic removal from groundwater of the Chaco-Pampean Plain (Argentina) using natural geological materials as adsorbents. J Environ Sci Health, Part A 46(11):1297–1310
Singh R, Singh S, Parihar P, Singh VP, Prasad SM (2015) Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol Environ Saf 112:247–270
Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, Goessler W, Pollak J, Silbergeld EK, Howard BV (2013) Association between exposure to low to moderate arsenic levels and incident cardiovascular disease: a prospective cohort study. Ann Intern Med 159(10):649–659
James KA, Byers T, Hokanson JE, Meliker JR, Zerbe GO, Marshall JA (2015) Association between lifetime exposure to inorganic arsenic in drinking water and coronary heart disease in Colorado residents. Environ Health Perspect 123(2):128
Lemaire M, Silva LFN, Lemarié CA, Bolt AM, Molina MF, Krohn RM, Smits JE, Lehoux S, Mann KK (2015) Arsenic exposure increases monocyte adhesion to the vascular endothelium, a pro-atherogenic mechanism. PLoS ONE 10(9):e0136592
Gong G, Basom J, Mattevada S, Onger F (2015) Association of hypothyroidism with low-level arsenic exposure in rural West Texas. Environ Res 138:154–160
Sun G (2004) Arsenic contamination and arsenicosis in China. Toxicol Appl Pharmacol 198(3):268–271
Vibol S, Hashim JH, Sarmani S (2015) Neurobehavioral effects of arsenic exposure among secondary school children in the Kandal Province, Cambodia. Environ Res 137:329–337
Bardach A, Ciapponi A, Soto N, Chaparro M, Calderon M, Briatore A, Cadoppi N, Tassara R, Litter M (2015) Epidemiology of chronic disease related to arsenic in Argentina: a systematic review. Sci Total Environ 538:802–816
Arain MB, Kazi TG, Baig JA, Afridi HI, Brehman KD, Panhwar H, Arain SS (2015) Co-exposure of arsenic and cadmium through drinking water and tobacco smoking: risk assessment on kidney dysfunction. Environ Sci Pollut Res 22(1):350–357
Tavakkoli N, Habibollahi S, Amini Tehrani S (2012) Modified activated carbon as solid phase extraction adsorbent for the preconcentration and determination of trace As (III) in environmental samples by graphite furnace atomic absorption spectrometry. Chin J Chem 30(3):665–669
Cacho F, Lauko L, Manova A, Beinrohr E (2012) On-line electrochemical pre-concentration of arsenic on a gold coated porous carbon electrode for graphite furnace atomic absorption spectrometry. J Anal At Spectrom 27(4):695–699
Trang PTK, Berg M, Viet PH, Mui NV, van der Meer JR (2005) Bacterial bioassay for rapid and accurate analysis of arsenic in highly variable groundwater samples. Environ Sci Technol 39(19):7625–7630
Thomas P, Sniatecki K (1995) Determination of trace amounts of arsenic species in natural waters by high-performance liquid chromatography–inductively coupled plasma mass spectrometry. J Anal At Spectrom 10(9):615–618
Arslan Y, Yildirim E, Gholami M, Bakirdere S (2011) Lower limits of detection in speciation analysis by coupling high-performance liquid chromatography and chemical-vapor generation. TrAC Trends Anal Chem 30(4):569–585
Mardegan A, Scopece P, Lamberti F, Meneghetti M, Moretto L, Ugo P (2012) Electroanalysis of trace inorganic arsenic with gold nanoelectrode ensembles. Electroanalysis 24(4):798–806
Lan Y, Luo H, Ren X, Wang Y, Wang L (2012) Glassy carbon electrode modified with citrate stabilized gold nanoparticles for sensitive arsenic (III) detection. Anal Lett 45(10):1184–1196
Thotiyl MO, Basit H, Sánchez JA, Goyer C, Coche-Guerente L, Dumy P, Sampath S, Labbé P, Moutet J-C (2012) Multilayer assemblies of polyelectrolyte–gold nanoparticles for the electrocatalytic oxidation and detection of arsenic (III). J Colloid Interface Sci 383(1):130–139
Gu T, Bu L, Huang Z, Liu Y, Tang Z, Liu Y, Huang S, Xie Q, Yao S, Tu X (2013) Dual-signal anodic stripping voltammetric determination of trace arsenic (III) at a glassy carbon electrode modified with internal-electrolysis deposited gold nanoparticles. Electrochem Commun 33:43–46
Huang J-F, Chen H-H (2013) Gold-nanoparticle-embedded nafion composite modified on glassy carbon electrode for highly selective detection of arsenic (III). Talanta 116:852–859
Luong JH, Lam E, Male KB (2014) Recent advances in electrochemical detection of arsenic in drinking and ground waters. Anal Methods 6(16):6157–6169
Nambiar SR, Prathish KP, Karthik G, Rao TP (2011) Hybrid gold atomic cluster–cobalt oxide scaffolds for dual tandem electrocatalytic sensing of cysteine. Biosens Bioelectron 26(9):3920–3926
Boyen H-G, Kästle G, Weigl F, Koslowski B, Dietrich C, Ziemann P, Spatz J, Riethmüller S, Hartmann C, Möller M (2002) Oxidation-resistant gold-55 clusters. Science 297(5586):1533–1536
Zhan H, Li J, Liu Z, Zheng Y, Jing Y (2015) A highly sensitive electrochemical OP biosensor based on electrodeposition of Au–Pd bimetallic nanoparticles onto a functionalized graphene modified glassy carbon electrode. Anal Methods 7(9):3903–3911
L-u Rahman, Shah A, Lunsford SK, Han C, Nadagouda MN, Sahle-Demessie E, Qureshi R, Khan MS, Kraatz H-B, Dionysiou DD (2015) Monitoring of 2-butanone using a Ag–Cu bimetallic alloy nanoscale electrochemical sensor. RSC Adv 5(55):44427–44434
Shen C, Su J, Li X, Luo J, Yang M (2015) Electrochemical sensing platform based on Pd–Au bimetallic cluster for non-enzymatic detection of glucose. Sens Actuators, B 209:695–700
Cui X, Wu S, Li Y, Wan GG (2015) Sensing hydrogen peroxide using a glassy carbon electrode modified with in situ electrodeposited platinum-gold bimetallic nanoclusters on a graphene surface. Microchim Acta 182(1–2):265–272
Moghimi N, Mohapatra M, Leung KT (2015) Bimetallic nanoparticles for arsenic detection. Anal Chem 87:5546–5552
Li J, Li X, Zhai H-J, Wang L-S (2003) Au20: a tetrahedral cluster. Science 299(5608):864–867
Zhang C, Yoon B, Landman U (2007) Predicted oxidation of CO catalyzed by au nanoclusters on a thin defect-free MgO film supported on a Mo (100) surface. J Am Chem Soc 129(8):2228–2229
Kauffman DR, Sorescu DC, Schofield DP, Allen BL, Jordan KD, Star A (2010) Understanding the sensor response of metal-decorated carbon nanotubes. Nano Lett 10(3):958–963
Wang J, Wang G, Zhao J (2003) Structures and electronic properties of Cu 20, Ag 20, and Au 20 clusters with density functional method. Chem Phys Lett 380(5):716–720
King RB, Chen Z, PvR Schleyer (2004) Structure and bonding in the omnicapped truncated tetrahedral Au20 cluster: analogies between gold and carbon cluster chemistry. Inorg Chem 43(15):4564–4566
Fernández EM, Soler JM, Garzón IL, Balbás LC (2004) Trends in the structure and bonding of noble metal clusters. Phys Rev B 70(16):165403
Aikens CM, Schatz GC (2006) TDDFT studies of absorption and SERS spectra of pyridine interacting with Au20. J Phys Chem A 110(49):13317–13324
Le HT, Lang SM, De Haeck J, Lievens P, Janssens E (2012) Carbon monoxide adsorption on neutral and cationic vanadium doped gold clusters. Phys Chem Chem Phys 14(26):9350–9358
De Haeck J, Veldeman N, Claes P, Janssens E, Andersson M, Lievens P (2011) Carbon monoxide adsorption on silver doped gold clusters. J Phys Chem A 115(11):2103–2109
Jena NK, Chandrakumar K, Ghosh SK (2009) Theoretical investigation on the structure and electronic properties of hydrogen-and alkali-metal-doped gold clusters and their interaction with CO: enhanced reactivity of hydrogen-doped gold clusters. J Phys Chem C 113(41):17885–17892
Nhat PV, Tai TB, Nguyen MT (2012) Theoretical study of AunV-CO, n = 1–14: the dopant vanadium enhances CO adsorption on gold clusters. J Chem Phys 137(16):164312
Beletskaya AV, Pichugina DA, Shestakov AF, Kuz’menko NE (2013) Formation of H2O2 on Au20 and Au19Pd clusters: understanding the structure effect on the atomic level. J Phys Chem A 117(31):6817–6826
Negishi Y, Kurashige W, Niihori Y, Iwasa T, Nobusada K (2010) Isolation, structure, and stability of a dodecanethiolate-protected Pd1Au24 cluster. Phys Chem Chem Phys 12(23):6219–6225
Xie S, Tsunoyama H, Kurashige W, Negishi Y, Tsukuda T (2012) Enhancement in aerobic alcohol oxidation catalysis of Au25 clusters by single Pd atom doping. ACS Catal 2(7):1519–1523
Negishi Y, Igarashi K, Munakata K, Ohgake W, Nobusada K (2012) Palladium doping of magic gold cluster Au 38 (SC2 H 4 Ph) 24: formation of Pd 2 Au 36 (SC 2 H 4 Ph) 24 with higher stability than Au 38 (SC 2 H 4 Ph) 24. Chem Commun 48(5):660–662
Qian H, Barry E, Zhu Y, Jin R (2011) Doping 25-atom and 38-atom gold nanoclusters with palladium. Acta Phys Chim Sin 27(3):513–519
Koyasu K, Naono Y, Akutsu M, Mitsui M, Nakajima A (2006) Photoelectron spectroscopy of binary Au cluster anions with a doped metal atom: Au n M − (n = 2–7), M = Pd, Ni, Zn, Cu, and Mg. Chem Phys Lett 422(1):62–66
Ghanty TK, Banerjee A, Chakrabarti A (2009) Structures and the Electronic Properties of Au19X Clusters (X = Li, Na, K, Rb, Cs, Cu, and Ag). J Phys Chem C 114(1):20–27
Cao Y, Li X (2014) Adsorption of graphene for the removal of inorganic pollutants in water purification: a review. Adsorption 20(5–6):713–727
Cortés-Arriagada D, Toro-Labbé A (2015) Improving As (iii) adsorption on graphene based surfaces: impact of chemical doping. Phys Chem Chem Phys 17(18):12056–12064
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865
Hehre WJ, Ditchfield R, Pople JA (1972) Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56(5):2257–2261
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J Chem Phys 77(7):3654–3665
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys 82(1):299–310
Ehlers A, Böhme M, Dapprich S, Gobbi A, Höllwarth A, Jonas V, Köhler K, Stegmann R, Veldkamp A, Frenking G (1993) A set of f-polarization functions for pseudo-potential basis sets of the transition metals Sc-Cu, Y-Ag and La-Au. Chem Phys Lett 208(1):111–114
Roy LE, Hay PJ, Martin RL (2008) Revised basis sets for the LANL effective core potentials. J Chem Theory Comput 4(7):1029–1031
Schuchardt KL, Didier BT, Elsethagen T, Sun L, Gurumoorthi V, Chase J, Li J, Windus TL (2007) Basis set exchange: a community database for computational sciences. J Chem Inf Model 47(3):1045–1052
Mondal K, Banerjee A, Ghanty TK (2014) Structural and chemical properties of subnanometer-sized bimetallic Au19Pt cluster. J Phys Chem C 118(22):11935–11945
Cortés-Arriagada D, Oyarzún MP, Sanhueza L, Toro-Labbé A (2015) Binding of trivalent arsenic onto the tetrahedral Au20 and Au19Pt clusters: implications in adsorption and sensing. J Phys Chem A 119(26):6909–6918
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32(7):1456–1465
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132(15):154104
Boys SF, Bernardi Fd (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19(4):553–566
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision B.01. Gaussian 09 Revision B01:Wallingford CT
Neese F (2012) The ORCA program system. Wiley Interdiscip Rev: Comput Mol Sci 2(1):73–78
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Pierce ML, Moore CB (1982) Adsorption of arsenite and arsenate on amorphous iron hydroxide. Water Res 16(7):1247–1253
Cutter GA (1992) Kinetic controls on metalloid speciation in seawater. Mar Chem 40(1):65–80
Addison AW, Rao TN, Reedijk J, van Rijn J, Verschoor GC (1984) Synthesis, structure, and spectroscopic properties of copper (II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1, 7-bis (N-methylbenzimidazol-2′-yl)-2, 6-dithiaheptane] copper (II) perchlorate. J Chem Soc, Dalton Trans 7:1349–1356
Bianconi A, Saini N, Lanzara A, Missori M, Rossetti T, Oyanagi H, Yamaguchi H, Oka K, Ito T (1996) Determination of the Local Lattice Distortions in the Cu O 2 Plane of L a 1.85 S r 0.15 Cu O 4. Phys Rev Lett 76(18):3412
Sareen N, Singh S, Bhattacharya S (2014) A Cu (II) mediated new desulfurization pathway involving elimination of ethylene sulfide. Dalton Trans 43(12):4635–4638
Churchill MR, Missert JR (1981) Molecules with an M4X4 core. 12. Disruption of tetrameric (triphenylarsine) copper (I) iodide in acetonitrile solution. Crystal structure of [(AsPh3)(MeCN) CuI] 2. Inorg Chem 20(2):619–621
Churchill MR, Youngs WJ (1979) Molecules with an M4X4 core. Part 11. The unexpected isolation of the cubane-like isomer of tetrameric (triphenylarsine) copper (I) iodide. Crystal structure of [(AsPh3) CuI] 4. C6H6. Inorg Chem 18(4):1133–1138
Vignolle J, Gornitzka H, Donnadieu B, Bourissou D, Bertrand G (2008) Palladium–oxygen and palladium–arene interactions in complexes derived from biaryl aminocarbenes: comparison with biaryl phosphanes. Angew Chem Int Ed 47(12):2271–2274
Alfredsson M, Catlow CRA (2001) Modelling of Pd and Pt supported on the 111 and 011 surfaces of cubic-ZrO 2. Phys Chem Chem Phys 3(18):4129–4140
Ma M, Lu R, Pullarkat SA, Deng W, Leung P-H (2010) Steric effects on the control of endo/exo-selectivity in the asymmetric cycloaddition reaction of 3,4-dimethyl-1-phenylarsole. Dalton Trans 39(23):5453–5461
Yin J, Buchwald SL (2002) Pd-catalyzed intermolecular amidation of aryl halides: the discovery that xantphos can be trans-chelating in a palladium complex. J Am Chem Soc 124(21):6043–6048
Oliveira AF, Ladeira ACQ, Ciminelli VS, Heine T, Duarte HA (2006) Structural model of arsenic (III) adsorbed on gibbsite based on DFT calculations. J Mol Struct THEOCHEM 762(1):17–23
Blanchard M, Wright K, Gale JD, Catlow CRA (2007) Adsorption of As (OH) 3 on the (001) surface of FeS2 pyrite: a quantum-mechanical DFT Study. J Phys Chem C 111(30):11390–11396
Wei Z, Liang K, Wu Y, Zou Y, Zuo J, Arriagada DC, Pan Z, Hu G (2016) The effect of pH on the adsorption of arsenic(III) and arsenic(V) at the TiO2 anatase [1 0 1] surface. J Colloid Interface Sci 462:252–259. doi:10.1016/j.jcis.2015.10.018
Kryachko E, Remacle F (2007) The magic gold cluster Au20. Int J Quantum Chem 107(14):2922–2934
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132(18):6498–6506
Bader RF (1985) Atoms in molecules. Acc Chem Res 18(1):9–15
Kryachko E, Remacle F (2005) Three-gold clusters form nonconventional hydrogen bonds O–H···Au and N–H···Au with formamide and formic acid. Chem Phys Lett 404(1):142–149
Kryachko E, Karpfen A, Remacle F (2005) Nonconventional hydrogen bonding between clusters of gold and hydrogen fluoride. J Phys Chem A 109(32):7309–7318
Kryachko ES (2008) Where gold meets a hydrogen bond? J Mol Struct 880(1):23–30
Kacprzak KA, Lehtovaara L, Akola J, Lopez-Acevedo O, Häkkinen H (2009) A density functional investigation of thiolate-protected bimetal PdAu 24 (SR) 18 z clusters: doping the superatom complex. Phys Chem Chem Phys 11(33):7123–7129
Ferguson JF, Gavis J (1972) A review of the arsenic cycle in natural waters. Water Res 6(11):1259–1274
Klamt A (2011) The COSMO and COSMO-RS solvation models. Wiley Interdiscip Rev Comput Mol Sci 1(5):699–709
Acknowledgments
This work was supported by the Project: FONDECYT/Postdoctorado N° 3140314, FONDECYT 1130072 and ICM Grant N° 120082.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles “CHITEL 2015 - Torino - Italy”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cortés-Arriagada, D., Toro-Labbé, A. Insights into the use of Au19Cu and Au19Pd clusters for adsorption of trivalent arsenic. Theor Chem Acc 135, 52 (2016). https://doi.org/10.1007/s00214-016-1825-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1825-9