Abstract
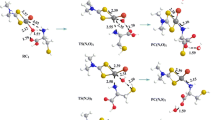
The reaction mechanism of cationic Au(I) N-heterocyclic carbine (NHC) complexes [(R2Im)2Au]+ (R = Me, Et, i-Pr, and n-Pr) binding to Cys and Sec residues for the monofunctional and bifunctional substitution reaction was studied using the density functional theory with the B3LYP functional. The optimized geometries reveal the transition state configuration of Au(I) complex exhibited trigonal planar configuration. Over-investigation of the activation energies of all the complexes demonstrated that Me group complexes were more favorable in monofunctional substitution reaction, while the bifunctional substitution reaction preferred i-Pr group complexes. In addition, the reaction of Au(I) complexes binding to the active site Sec selenol was stronger than to the active site Cys thiols. Moreover, to consider the environment effect, we employed the isoelectric focusing polarizable continuum model to calculate the single-point energy in dependence of the dielectric constant ɛ, observing the environment was a weeny impact on the binding of Au(I) NHC complexes to its intracellular targets.

















Similar content being viewed by others
References
Lima JC, Rodriguez L (2011) Anti-Cancer Agents Med Chem 11:921
Zhou LX (2009) J Phys Chem B 113:2110
Jakupec MA, Galanski M, Keppler BK (2003) Rev Physiol Biochem Pharmacol 146:1
Fuertes MA, Alonso C, Perez JM (2003) Chem Rev 103:645
Perez RP (1998) Eur J Cancer 34:1535
Alberto ME, Lucas MF, Pavelka M, Russo N (2008) J Phys Chem B 112:10765
Bruijnincx PCA, Sadler PJ (2008) Curr Opin Chem Biol 12:197
Ronconi L, Sadler PJ (2007) Coord Chem Rev 251:1633
Ott I, Gust R (2007) Arch Pharm Chem Life Sci 340:117
Meggers E (2007) Curr Opin Chem Biol 11:287
Zhao HL, Zhou LX (2012) Comput Theor Chem 979:22
Rubbiani R, Kitanovic I, Alborzinia H, Can S, Kitanovic A, Onambele LA, Stefanopoulou M, Geldmacher Y, Sheldrick WS, Wolber G, Prokop A, Wölfl S, Ott I (2010) J Med Chem 53:8608
Anestal K, Arner ES (2003) J Biol Chem 278:15966
Marzano C, Gandin V, Folda A, Scutari G, Bindoli A, Rigobello MA (2007) Free Radic Biol Med 42:872
Liao JZ, Zhao HL, Zhou LX (2014) Comput Theor Chem 1048:84
Berners-Price SJ, Filipovska A (2011) Metallomics 3:86
Sharma RP, Smillie J, Palmer DG (1985) Pharmacology 30:115
Fernández GA, Vela-Gurovic MS, Olivera NL, Chopa AB, Silbestri GF (2014) J Inorg Biochem 135:54
Alex J, Ghosh P (2010) Dalton Trans 39:7183
Arduengo AJ, Harlow RL, Kline M (1991) J Am Chem Soc 113:361
Baker MV, Barnard PJ, Berners-Price SJ (2006) Dalton Trans 30:3708
Gautier A, Cisnetti F (2012) Metallomics 4:23
Oehninger L, Rubbiani R, Ott I (2013) Dalton Trans 42:3269
Hickey JL, Ruhayel RA, Barnard PJ (2008) J Am Chem Soc 130:12570
Liu Wk, Gust R (2013) Chem Soc Rev 42:755
Green DR (2004) Sci 305:626
Galluzzi L, Larochette N, Zamzami N, Kroemer G (2006) Oncogene 25:4812
Fantin VR, Leder P (2006) Oncogene 25:4787
Lincoln DT, Ali Emadi EM, Tonissen KF, Clarke FM (2003) Anticancer Res 23:2425
Rundolf AK, Arner ES (2004) Antioxid Redox Signal 6:41
Ott I (2009) Coord Chem Rev 253:1670
Nobili S, Mini E, Landini I, Gabbiani C, Casini A, Messori L (2010) Med Res Rev 30:550
Bernardi P (1999) Physiol Rev 79:1127
Debatin KM, Poncet D, Kroemer G (2002) Oncogene 21:8786
Makin G, Dive C (2003) Trends Mol Med 9:251
Dias N, Bailly C (2005) Biochem Pharmacol 70:1
Urig S, Fritz-Wolf K, Reau R (2006) Angew Chem Int Ed 45:1881
Frisch MJ et al (2010) Gaussian 09 (Revision C.01). Gaussian, Inc., Wallingford
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Wadt WR, Hay PJ (1985) J Chem Phys 82:284
Hay PJ, Wadt WR (1985) J Chem Phys 82:270
Hay PJ, Wadt WR (1985) J Chem Phys 82:299
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Mennucci B, Tomasi J (1997) J Chem Phys 106:5151
Mennucci B, Cances E (1997) J Phys Chem B 101:10506
Tomasi J, Mennucci B, Cances E (1999) J Mol Struct (THEOCHEM) 464:211
Deubel DV (2004) J Am Chem Soc 126:5999
Archontis G, Simonson T (2001) J Am Chem Soc 123:11047
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 21271088).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, X., Zhou, L. A theoretical study on the anticancer drug Au(I) N-heterocyclic carbine complexes [(R2Im)2Au]+ (R = Me, Et, i-Pr, and n-Pr) binding to cysteine and selenocysteine residues. Theor Chem Acc 135, 30 (2016). https://doi.org/10.1007/s00214-015-1776-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-015-1776-6