Abstract
Rationale
Although nicotine from cigarettes is delivered in puff-sized amounts, most preclinical and human intravenous (IV) nicotine studies have used bolus or continuous infusions.
Objectives
To determine the feasibility of a pulsed-nicotine infusion model in smokers.
Methods
Following overnight abstinence, 12 adult smokers underwent 5 laboratory sessions. Using a crossover design, in each session, participants were assigned to 1 of 5 conditions: (1) high/fast: 1.0 mg nicotine delivered over 5 pulsed-infusions, then 15 saline infusions; (2) high/slow: 1.0 mg nicotine delivered over 20 pulsed-infusions; (3) low/fast: 0.2 mg nicotine delivered over 5 pulsed-infusions, then 15 saline infusions; (4) low/slow: 0.2 mg nicotine delivered over 20 pulsed-infusions; and (5) placebo: Saline delivered over 20 pulsed-infusions. Subjective drug effects, urges to smoke, nicotine withdrawal, and cognitive performance were measured in each session.
Results
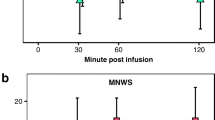
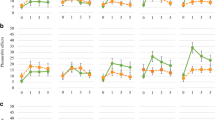
Both the high/fast and high/slow conditions were associated with greater “head rush” and “high” (p < 0.05). The high/fast condition also provided greater suppression of urges to smoke and nicotine withdrawal (p < 0.05), indexed by the Questionnaire of Urges to Smoke-Brief, and the Minnesota Nicotine Withdrawal Scale, respectively. The high/fast and high/slow conditions produced greater increases in heart rate (p < 0.01) than saline. Finally, there were no main effects of dosing conditions on cognitive performance, indexed by the continuous performance test.
Conclusions
These findings demonstrate the feasibility of pulsed-nicotine infusions to model nicotine delivery by smoking. This model could inform future studies testing novel smoking cessation therapies and tobacco regulatory studies testing the impact of nicotine reduction approaches.




Similar content being viewed by others
References
Bitzer ZT, Goel R, Reilly SM, Bhangu G, Trushin N, Foulds J, Muscat J, Richie JP Jr (2018) Emissions of free radicals, carbonyls, and nicotine from the NIDA Standardized Research Electronic Cigarette and comparison to similar commercial devices. Chem Res Toxicol 32:130–138
Carmines E, Gillman IG (2019) Comparison of the yield of very low nicotine content cigarettes to the top 100 United States brand styles. Beiträge zur Tabakforschung International/Contributions to Tobacco Research 28:253–266
Cox LS, Tiffany ST, Christen AG (2001) Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 3:7–16
De Aquino JP, MacLean RR, Gueorguieva R, DeVito EE, Eid T, Sofuoglu M (2022) Impact of delivery rate on the acute response to intravenous nicotine: a human laboratory study with implications for regulatory science. Addict Biol 27:e13161
DeVito EE, Herman AI, Waters AJ, Valentine GW, Sofuoglu M (2014) Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology 39:1431–1440
Ding YS, Richter P, Hearn B (2017) Chemical characterization of mainstream smoke from SPECTRUM variable nicotine research cigarettes. Tobacco Regulatory Science 3:81–94
El-Hellani A, Salman R, El-Hage R, Talih S, Malek N, Baalbaki R, Karaoghlanian N, Nakkash R, Shihadeh A, Saliba NA (2016) Nicotine and carbonyl emissions from popular electronic cigarette products: correlation to liquid composition and design characteristics. Nicotine Tob Res 20:215–223
El-Hellani A, Salman R, El-Hage R, Talih S, Malek N, Baalbaki R, Karaoghlanian N, Nakkash R, Shihadeh A, Saliba NA (2018) Nicotine and carbonyl emissions from popular electronic cigarette products: correlation to liquid composition and design characteristics. Nicotine Tob Res 20:215–223
Etter JF, Vu Duc T, Perneger TV (2000) Saliva cotinine levels in smokers and nonsmokers. Am J Epidemiol 151:251–258
Giniatullin R, Nistri A, Yakel JL (2005) Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci 28:371–378
Goldberg SR, Henningfield JE (1988) Reinforcing effects of nicotine in humans and experimental animals responding under intermittent schedules of iv drug injection. Pharmacol Biochem Behav 30:227–234
Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR (2004) Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology 175:134–142
Heishman SJ, Kleykamp BA, Singleton EG (2010) Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology 210:453–469
Hurst R, Rollema H, Bertrand D (2013) Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther 137:22–54
Jensen KP, DeVito EE, Herman AI, Valentine GW, Gelernter J, Sofuoglu M (2015) A CHRNA5 smoking risk variant decreases the aversive effects of nicotine in humans. Neuropsychopharmacology 40:2813–2821
Jensen KP, DeVito EE, Valentine G, Gueorguieva R, Sofuoglu M (2016) Intravenous nicotine self-administration in smokers: Dose–response function and sex differences. Neuropsychopharmacology 41:2034–2040
Jensen KP, Valentine G, Gueorguieva R, Sofuoglu M (2020) Differential effects of nicotine delivery rate on subjective drug effects, urges to smoke, heart rate and blood pressure in tobacco smokers. Psychopharmacology 237:1359–1369
Jones HE, Garrett BE, Griffiths RR (1999) Subjective and physiological effects of intravenous nicotine and cocaine in cigarette smoking cocaine abusers. J Pharmacol Exp Ther 288:188–197
Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB (2003) Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res 5:673–679
MacLean RR, DeVito EE, Eid T, Parida S, Gueorguieva R, Sofuoglu M (2021a) Threshold dose for intravenous nicotine self-administration in young adult non-dependent smokers. Psychopharmacology 238:2083–2090
MacLean RR, Gueorguieva R, DeVito EE, Peltier MR, Parida S, Sofuoglu M (2021c) The effects of inhaled flavors on intravenous nicotine. Exp Clin Psychopharmacol 29:615–624
MacLean RR, DeVito EE, Eid T, Parida S, Gueorguieva R, Sofuoglu M (2021b) Threshold dose for intravenous nicotine self-administration in young adult non-dependent smokers. Psychopharmacology 238(8):2083–2090. Chicago
Marks MJ, Stitzel JA, Collins AC (1987) Influence of kinetics of nicotine administration on tolerance development and receptor levels. Pharmacol Biochem Behav 27:505–512
Myers CS, Taylor RC, Moolchan ET, Heishman SJ (2008) Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology 33:588–598
Reeves DL, Bleiberg J, Roebuck-Spencer T, Cernich AN, Schwab K, Ivins B, Salazar AM, Harvey SC, Brown FH Jr, Warden D (2006) Reference values for performance on the Automated Neuropsychological Assessment Metrics V3.0 in an active duty military sample. Mil Med 171:982–994
Reitsma MB, Kendrick PJ, Ababneh E, Abbafati C, Abbasi-Kangevari M, Abdoli A, Abedi A, Abhilash E, Abila DB, Aboyans V (2021) Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. The Lancet
Rose JE, Behm FM, Westman EC, Coleman RE (1999a) Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction. Drug Alcohol Depend 56:99–107
Rose JE, Behm FM, Westman EC, Bates JE (2003a) Mecamylamine acutely increases human intravenous nicotine self-administration. Pharmacol Biochem Behav 76:307–313
Rose JE, Behm FM, Westman EC, Bates JE, Salley A (2003b) Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacol Biochem Behav 76:243–250
Rose JE, Salley A, Behm FM, Bates JE, Westman EC (2010a) Reinforcing effects of nicotine and non-nicotine components of cigarette smoke. Psychopharmacology 210:1–12
Rupprecht LE, Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC (2015) Behavioral mechanisms underlying nicotine reinforcement. The neuropharmacology of nicotine dependence: 19–53.
Semenova S, Jin X, McClure-Begley TD, Tadman MP, Marks MJ, Markou A (2018) Differential effects of withdrawal from intermittent and continuous nicotine exposure on reward deficit and somatic aspects of nicotine withdrawal and expression of α4β2* nAChRs in Wistar male rats. Pharmacol Biochem Behav 171:54–65
Shihadeh A, Eissenberg T (2015) Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux. Nicotine Tob Res 17:158–162
Sofuoglu M, Yoo S, Hill KP, Mooney M (2008) Self-administration of intravenous nicotine in male and female cigarette smokers. Neuropsychopharmacology 33:715–720
Sorge RE, Clarke PB (2009) Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. J Pharmacol Exp Ther 330:633–640
St. Helen G, Havel C, Dempsey DA, Jacob P III, Benowitz NL (2016) Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 111:535–544
Tiffany ST, Drobes DJ (1991) The development and initial validation of a questionnaire on smoking urges. Br J Addict 86:1467–1476
Toll BA, O’Malley SS, McKee SA, Salovey P, Krishnan-Sarin S (2007) Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychol Addict Behav 21:216–225
Walton K, Hampson A (2017) The NIDA Standardized Research E-Cigarette (SREC)
Funding
The authors received funding from the US Department of Veteran Affairs, VISN-1 Mental Illness Research, Education, and Clinical Center (MIRECC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
213_2022_6162_MOESM2_ESM.pdf
Supplementary file2 (PDF 389 kb) Online Resource 2. Subjective nicotine effects from pulsed-nicotine infusions. Mean scores for the DEQ ratings of a) “ like”, b) “want more”, c) “good drug effect”, and d) “bad drug effect” in response to 5 different delivery conditions: 1) High/Fast: 1.0 mg nicotine delivered over 5 pulsed-infusions; 2) High/Slow: 1.0 mg nicotine delivered over 20 pulsed-infusions; 3) Low/Fast: 0.2 mg nicotine delivered over 5 pulsed-infusions; 4) Low/Slow: 0.2 mg nicotine delivered over 20 pulsed-infusions; and 5) Placebo: Saline delivered over 20 pulsed-infusions. Error bars reflect the standard error of the mean.
213_2022_6162_MOESM4_ESM.pdf
Supplementary file4 (PDF 86 kb) Online Resource 4. Impact of pulsed-nicotine infusions on cognitive performance. The mean change in Continuous Performance Test (CPT) throughput score, which reflects both speed and accuracy, in response to 5 different delivery conditions: 1) High/Fast: 1.0 mg nicotine delivered over 5 pulsed-infusions; 2) High/Slow: 1.0 mg nicotine delivered over 20 pulsed-infusions; 3) Low/Fast: 0.2 mg nicotine delivered over 5 pulsed-infusions; 4) Low/Slow: 0.2 mg nicotine delivered over 20 pulsed-infusions; and 5) Placebo: Saline delivered over 20 pulsed-infusions. Error bars reflect the standard error of the mean.
Rights and permissions
About this article
Cite this article
De Aquino, J.P., DeVito, E.E., Xie, C. et al. Development of pulsed intravenous nicotine infusions as a model for inhaled nicotine in humans. Psychopharmacology 239, 2809–2818 (2022). https://doi.org/10.1007/s00213-022-06162-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06162-0