Abstract
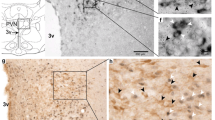
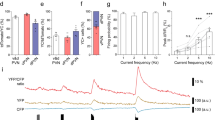
Relaxin-3/RXFP3 signalling is proposed to be involved in the neuromodulatory control of arousal- and stress-related neural circuits. Furthermore, previous studies in rats have led to the proposal that relaxin-3/RXFP3 signalling is associated with activation of the hypothalamic-pituitary-adrenal axis, but direct evidence for RXFP3-related actions on the activity of hypothalamic corticotropin-releasing hormone (CRH) neurons is lacking. In this study, we investigated characteristics of the relaxin-3/RXFP3 system in mouse hypothalamus. Administration of an RXFP3 agonist (RXFP3-A2) intra-cerebroventricularly or directly into the paraventricular nucleus of hypothalamus (PVN) of C57BL/6J mice did not alter corticosterone levels. Similarly, there were no differences between serum corticosterone levels in Rxfp3 knockout (C57BL/6JRXFP3TM1) and wild-type mice at baseline and after stress, despite detection of the predicted stress-induced increases in serum corticosterone. We examined the nature of the relaxin-3 innervation of PVN in wild-type mice and in Crh-IRES-Cre;Ai14 mice that co-express the tdTomato fluorophore in CRH neurons, identifying abundant relaxin-3 fibres in the peri-PVN region, but only sparse fibres associated with densely packed CRH neurons. In whole-cell voltage-clamp recordings of tdTomato-positive CRH neurons in these mice, we observed a reduction in sEPSC frequency following local application of RXFP3-A2, consistent with an activation of RXFP3 on presynaptic glutamatergic afferents in the PVN region. These studies clarify the relationship between relaxin-3/RXFP3 inputs and CRH neurons in mouse PVN, with implications for the interpretation of current and previous in vivo studies and future investigations of this stress-related signalling network in normal and transgenic mice, under normal and pathological conditions.





Similar content being viewed by others
References
Adams JM, Otero-Corchon V, Hammond GL, Veldhuis JD, Qi N, Low MJ (2015) Somatostatin is essential for the sexual dimorphism of GH secretion, corticosteroid-binding globulin production, and corticosterone levels in mice. Endocrinology 156:1052–1065
Ang SY, Hutchinson DS, Evans BA, Hossain MA, Patil N, Bathgate RA, Kocan M, Summers RJ (2017) The actions of relaxin family peptides on signal transduction pathways activated by the relaxin family peptide receptor RXFP4. Naunyn Schmiedeberg's Arch Pharmacol 390:105–111
Backstrom T, Winberg S (2013) Central corticotropin releasing factor and social stress. Front Neurosci 7:117
Bains JS, Wamsteeker Cusulin JI, Inoue W (2015) Stress-related synaptic plasticity in the hypothalamus. Nat Rev Neurosci 16:377–388
Banerjee A, Shen PJ, Ma S, Bathgate RAD, Gundlach AL (2010) Swim stress excitation of nucleus incertus and rapid induction of relaxin-3 expression via CRF1 activation. Neuropharmacology 58:145–155
Bathgate RAD, Lin F, Hanson NF, Otvos L Jr, Guidolin A, Giannakis C, Bastiras S, Layfield SL, Ferraro T, Ma S, Zhao C, Gundlach AL, Samuel CS, Tregear GW, Wade JD (2006) Relaxin-3: improved synthesis strategy and demonstration of its high-affinity interaction with the relaxin receptor LGR7 both in vitro and in vivo. Biochemistry 45:1043–1053
Bell ME, Bhatnagar S, Akana SF, Choi S, Dallman MF (2000) Disruption of arcuate/paraventricular nucleus connections changes body energy balance and response to acute stress. J Neurosci 20:6707–6713
Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, Toga AW, Dong HW (2012) Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol 520:6–33
Blasiak A, Blasiak T, Lewandowski MH, Hossain MA, Wade JD, Gundlach AL (2013) Relaxin-3 innervation of the intergeniculate leaflet of the rat thalamus - neuronal tract-tracing and in vitro electrophysiological studies. Eur J Neurosci 37:1284–1294
Bodnar RJ, Klein GE (2004) Endogenous opiates and behavior: 2003. Peptides 25:2205–2256
Van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der Gugten J, Olivier B (2006) Mouse strain differences in autonomic responses to stress. Genes Brain Behav 5:139–149
Bonnavion P, Mickelsen LE, Fujita A, de Lecea L, Jackson AC (2016) Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. J Physiol 594:6443–6462
Borroto-Escuela DO, Agnati LF, Bechter K, Jansson A, Tarakanov AO, Fuxe K (2015) The role of transmitter diffusion and flow versus extracellular vesicles in volume transmission in the brain neural-glial networks. Philos Trans R Soc Lond Ser B Biol Sci 370:20140183
Boudaba C, Schrader LA, Tasker JG (1997) Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol 77:3396–3400
Calvez J, Lenglos C, de Avila C, Guevremont G, Timofeeva E (2015) Differential effects of central administration of relaxin-3 on food intake and hypothalamic neuropeptides in male and female rats. Genes Brain Behav 14:550–563
Chen J, Kuei C, Sutton SW, Bonaventure P, Nepomuceno D, Eriste E, Sillard R, Lovenberg TW, Liu C (2005) Pharmacological characterization of relaxin-3/INSL7 receptors GPCR135 and GPCR142 from different mammalian species. J Pharmacol Exp Ther 312:83–95
Csaki A, Kocsis K, Halasz B, Kiss J (2000) Localization of glutamatergic/aspartatergic neurons projecting to the hypothalamic paraventricular nucleus studied by retrograde transport of [3H]D-aspartate autoradiography. Neuroscience 101:637–655
Daniel SE, Rainnie DG (2016) Stress modulation of opposing circuits in the bed nucleus of the stria terminalis. Neuropsychopharmacology 41:103–125
DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV (2002) The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav 71:469–480
Fekete C, Sarkar S, Lechan RM (2005) Relative contribution of brainstem afferents to the cocaine- and amphetamine-regulated transcript (CART) innervation of thyrotropin-releasing hormone synthesizing neurons in the hypothalamic paraventricular nucleus (PVN). Brain Res 1032:171–175
Fernandes GA, Perks P, Cox NK, Lightman SL, Ingram CD, Shanks N (2002) Habituation and cross-sensitization of stress-induced hypothalamic-pituitary-adrenal activity: effect of lesions in the paraventricular nucleus of the thalamus or bed nuclei of the stria terminalis. J Neuroendocrinol 14:593–602
Flak JN, Myers B, Solomon MB, McKlveen JM, Krause EG, Herman JP (2014) Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. Eur J Neurosci 39:1903–1911
Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB (2012) Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology 37:27–38
Fox JH, Lowry CA (2013) Corticotropin-releasing factor-related peptides, serotonergic systems, and emotional behavior. Front Neurosci 7:169
Fuzesi T, Wittmann G, Lechan RM, Liposits Z, Fekete C (2009) Noradrenergic innervation of hypophysiotropic thyrotropin-releasing hormone-synthesizing neurons in rats. Brain Res 1294:38–44
Fuzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS (2016) Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun 7:11937
Gadek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J (2013) Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep 65:1655–1662
Ganella DE, Ryan PJ, Bathgate RAD, Gundlach AL (2012) Increased feeding and body weight gain in rats after acute and chronic activation of RXFP3 by relaxin-3 and receptor-selective peptides: functional and therapeutic implications. Behav Pharmacol 23:516–525
Ganella DE, Callander GE, Ma S, Bye CR, Gundlach AL, Bathgate RAD (2013) Modulation of feeding by chronic rAAV expression of a relaxin-3 peptide agonist in rat hypothalamus. Gene Ther 20:703–716
Ghosal S, Nunley A, Mahbod P, Lewis AG, Smith EP, Tong J, D'Alessio DA, Herman JP (2015) Mouse handling limits the impact of stress on metabolic endpoints. Physiol Behav 150:31–37
Goncharova ND (2013) Stress responsiveness of the hypothalamic-pituitary-adrenal axis: age-related features of the vasopressinergic regulation. Front Endocrinol (Lausanne) 4:26
Goto M, Swanson LW, Canteras NS (2001) Connections of the nucleus incertus. J Comp Neurol 438:86–122
Gutierrez R, Lobo MK, Zhang F, de Lecea L (2011) Neural integration of reward, arousal, and feeding: recruitment of VTA, lateral hypothalamus, and ventral striatal neurons. IUBMB Life 63:824–830
Haidar M, Guèvremont G, Zhang C, Bathgate RAD, Timofeeva E, Smith CM, Gundlach AL (2017) Relaxin-3 inputs target hippocampal interneurons and deletion of hilar relaxin-3 receptors in ‘floxed-RXFP3’ mice impairs spatial memory. Hippocampus. doi:10.1002/hipo.22709
Herman JP, Flak J, Jankord R (2008) Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res 170:353–364
Hernandez VS, Vazquez-Juarez E, Marquez MM, Jauregui-Huerta F, Barrio RA, Zhang L (2015) Extra-neurohypophyseal axonal projections from individual vasopressin-containing magnocellular neurons in rat hypothalamus. Front Neuroanat 9:130
Higo S, Honda S, Iijima N, Ozawa H (2015) Mapping of kisspeptin receptor mRNA in the whole rat brain and its co-localization with oxytocin in the paraventricular nucleus. J Neuroendocrinol. doi:10.1111/jne.12356
Hosken IT, Sutton SW, Smith CM, Gundlach AL (2015) Relaxin-3 receptor (Rxfp3) gene knockout mice display reduced running wheel activity: implications for role of relaxin-3/RXFP3 signalling in sustained arousal. Behav Brain Res 278:167–175
Hossain MA, Man BC, Zhao C, Xu Q, Du XJ, Wade JD, Samuel CS (2011) H3 relaxin demonstrates antifibrotic properties via the RXFP1 receptor. Biochemistry 50:1368–1375
Hostetler CM, Ryabinin AE (2013) The CRF system and social behavior: a review. Front Neurosci 7:92
Hostinar CE, Sullivan RM, Gunnar MR (2014) Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull 140:256–282
Ju G, Swanson LW (1989) Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. J Comp Neurol 280:587–602
Ju G, Swanson LW, Simerly RB (1989) Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. J Comp Neurol 280:603–621
Kageyama K, Suda T (2009) Role and action in the pituitary corticotroph of corticotropin-releasing factor (CRF) in the hypothalamus. Peptides 30:810–816
Kania A, Czerw A, Grabowiecka A, Ávila CD, Blasiak T, Rajfur Z, Lewandowski MH, Hess G, Timofeeva E, Gundlach AL, Blasiak A (2017) Inhibition of oxytocin and vasopressin neuron activity in rat hypothalamic paraventricular nucleus by relaxin-3/RXFP3 signalling. J Physiol. doi:10.1113/JP273787
Kizawa H, Nishi K, Ishibashi Y, Harada M, Asano T, Ito Y, Suzuki N, Hinuma S, Fujisawa Y, Onda H, Nishimura O, Fujino M (2003) Production of recombinant human relaxin 3 in AtT20 cells. Regul Pept 113:79–84
De Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475
Kolasa M, Faron-Gorecka A, Kusmider M, Szafran-Pilch K, Solich J, Zurawek D, Gruca P, Papp M, Dziedzicka-Wasylewska M (2014) Differential stress response in rats subjected to chronic mild stress is accompanied by changes in CRH-family gene expression at the pituitary level. Peptides 61:98–106
Laflamme N, Barden N, Rivest S (1997) Corticotropin-releasing factor and glucocorticoid receptor (GR) gene expression in the paraventricular nucleus of immune-challenged transgenic mice expressing type II GR antisense ribonucleic acid. J Mol Neurosci 8:165–179
Lebow MA, Chen A (2016) Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry 21:450–463
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ et al (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445:168–176
Lenglos C, Mitra A, Guevremont G, Timofeeva E (2013) Sex differences in the effects of chronic stress and food restriction on body weight gain and brain expression of CRF and relaxin-3 in rats. Genes Brain Behav 12:370–387
Lenglos C, Mitra A, Guevremont G, Timofeeva E (2014) Regulation of expression of relaxin-3 and its receptor RXFP3 in the brain of diet-induced obese rats. Neuropeptides 48:119–132
Lenglos C, Calvez J, Timofeeva E (2015) Sex-specific effects of relaxin-3 on food intake and brain expression of corticotropin-releasing factor in rats. Endocrinology 156:523–533
Liposits Z, Phelix C, Paull WK (1986) Adrenergic innervation of corticotropin releasing factor (CRF)-synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A combined light and electron microscopic immunocytochemical study. Histochemistry 84:201–205
Liu C, Eriste E, Sutton S, Chen J, Roland B, Kuei C, Farmer N, Jornvall H, Sillard R, Lovenberg TW (2003) Identification of relaxin-3/INSL7 as an endogenous ligand for the orphan G-protein-coupled receptor GPCR135. J Biol Chem 278:50754–50764
Ma S, Shen PJ, Burazin TCD, Tregear GW, Gundlach AL (2006) Comparative localization of leucine-rich repeat-containing G-protein-coupled receptor-7 (RXFP1) mRNA and [33P]-relaxin binding sites in rat brain: restricted somatic co-expression a clue to relaxin action? Neuroscience 141:329–344
Ma S, Bonaventure P, Ferraro T, Shen PJ, Burazin TCD, Bathgate RAD, Liu C, Tregear GW, Sutton SW, Gundlach AL (2007) Relaxin-3 in GABA projection neurons of nucleus incertus suggests widespread influence on forebrain circuits via G-protein-coupled receptor-135 in the rat. Neuroscience 144:165–190
Ma S, Blasiak A, Olucha-Bordonau FE, Verberne AJ, Gundlach AL (2013) Heterogeneous responses of nucleus incertus neurons to corticotrophin-releasing factor and coherent activity with hippocampal theta rhythm in the rat. J Physiol 591:3981–4001
Ma S, Smith CM, Blasiak A, Gundlach AL (2016) Distribution, physiology and pharmacology of relaxin-3/RXFP3 systems in brain. Br J Pharmacol. doi:10.1111/bph.13659
Ma S, Allocca G, Ong-Pålsson EKE, Hawkes D, McDougall SJ, Williams SJ, Bathgate RAD, Gundlach AL (2017) Nucleus incertus promotes cortical desynchronization and behavioral arousal. Brain Struct Funct 222:515–537
McCarty R (2016) Learning about stress: neural, endocrine and behavioral adaptations. Stress 19:449–475
McGowan BM, Stanley SA, Smith KL, White NE, Connolly MM, Thompson EL, Gardiner JV, Murphy KG, Ghatei MA, Bloom SR (2005) Central relaxin-3 administration causes hyperphagia in male Wistar rats. Endocrinology 146:3295–3300
McGowan BM, Minnion JS, Murphy KG, Roy D, Stanley SA, Dhillo WS, Gardiner JV, Ghatei MA, Bloom SR (2014) Relaxin-3 stimulates the neuro-endocrine stress axis via corticotrophin-releasing hormone. J Endocrinol 221:337–346
Myers B, Scheimann JR, Franco-Villanueva A, Herman JP (2017) Ascending mechanisms of stress integration: implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neurosci Biobehav Rev 74:366–375
Nixon JP, Smale L (2005) Orexin fibers form appositions with Fos expressing neuropeptide-Y cells in the grass rat intergeniculate leaflet. Brain Res 1053:33–37
Nyuyki KD, Maloumby R, Reber SO, Neumann ID (2012) Comparison of corticosterone responses to acute stressors: chronic jugular vein versus trunk blood samples in mice. Stress 15:618–626
Oldfield BJ, Hou-Yu A, Silverman AJ (1985) A combined electron microscopic HRP and immunocytochemical study of the limbic projections to rat hypothalamic nuclei containing vasopressin and oxytocin neurons. J Comp Neurol 231:221–231
Olucha-Bordonau FE, Teruel V, Barcia-Gonzalez J, Ruiz-Torner A, Valverde-Navarro AA, Martinez-Soriano F (2003) Cytoarchitecture and efferent projections of the nucleus incertus of the rat. J Comp Neurol 464:62–97
Piccenna L, Shen PJ, Ma S, Burazin TCD, Gossen JA, Mosselman S, Bathgate RAD, Gundlach AL (2005) Localization of LGR7 gene expression in adult mouse brain using LGR7 knock-out/LacZ knock-in mice: correlation with LGR7 mRNA distribution. Ann N Y Acad Sci 1041:197–204
Ryan PJ, Buchler E, Shabanpoor F, Hossain MA, Wade JD, Lawrence AJ, Gundlach AL (2013a) Central relaxin-3 receptor (RXFP3) activation decreases anxiety- and depressive-like behaviours in the rat. Behav Brain Res 244:142–151
Ryan PJ, Kastman HE, Krstew EV, Rosengren KJ, Hossain MA, Churilov L, Wade JD, Gundlach AL, Lawrence AJ (2013b) Relaxin-3/RXFP3 system regulates alcohol-seeking. Proc Natl Acad Sci U S A 110:20789–20794
Shabanpoor F, Hossain MA, Ryan PJ, Belgi A, Layfield S, Kocan M, Zhang S, Samuel CS, Gundlach AL, Bathgate RAD, Separovic F, Wade JD (2012) Minimization of human relaxin-3 leading to high-affinity analogues with increased selectivity for relaxin-family peptide 3 receptor (RXFP3) over RXFP1. J Med Chem 55:1671–1681
Smith SM, Vale WW (2006) The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 8:383–395
Smith CM, Shen PJ, Banerjee A, Bonaventure P, Ma S, Bathgate RAD, Sutton SW, Gundlach AL (2010) Distribution of relaxin-3 and RXFP3 within arousal, stress, affective, and cognitive circuits of mouse brain. J Comp Neurol 518:4016–4045
Smith CM, Hosken IT, Sutton SW, Lawrence AJ, Gundlach AL (2012) Relaxin-3 null mutation mice display a circadian hypoactivity phenotype. Genes Brain Behav 11:94–104
Smith CM, Chua BE, Zhang C, Walker AW, Haidar M, Hawkes D, Shabanpoor F, Hossain MA, Wade JD, Rosengren KJ, Gundlach AL (2014a) Central injection of relaxin-3 receptor (RXFP3) antagonist peptides reduces motivated food seeking and consumption in C57BL/6J mice. Behav Brain Res 268:117–126
Smith CM, Walker AW, Hosken IT, Chua BE, Zhang C, Haidar M, Gundlach AL (2014b) Relaxin-3/RXFP3 networks: an emerging target for the treatment of depression and other neuropsychiatric diseases? Front Pharmacol 5:46
Sunn N, McKinley MJ, Oldfield BJ (2001) Identification of efferent neural pathways from the lamina terminalis activated by blood-borne relaxin. J Neuroendocrinol 13:432–437
Tanaka M, Iijima N, Miyamoto Y, Fukusumi S, Itoh Y, Ozawa H, Ibata Y (2005) Neurons expressing relaxin 3/INSL 7 in the nucleus incertus respond to stress. Eur J Neurosci 21:1659–1670
Tartar JL, King MA, Devine DP (2006) Glutamate-mediated neuroplasticity in a limbic input to the hypothalamus. Stress 9:13–19
Tortorella C, Neri G, Nussdorfer GG (2007) Galanin in the regulation of the hypothalamic-pituitary-adrenal axis (review). Int J Mol Med 19:639–647
Ueta Y, Fujihara H, Serino R, Dayanithi G, Ozawa H, Matsuda K, Kawata M, Yamada J, Ueno S, Fukuda A, Murphy D (2005) Transgenic expression of enhanced green fluorescent protein enables direct visualization for physiological studies of vasopressin neurons and isolated nerve terminals of the rat. Endocrinology 146:406–413
Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nature Rev Neurosci 10:397–409
Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP (2005) Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289:E823–E828
Vale W, Spiess J, Rivier C, Rivier J (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213:1394–1397
Walker AW, Smith CM, Chua BE, Krstew EV, Zhang C, Gundlach AL, Lawrence AJ (2015) Relaxin-3 receptor (RXFP3) signalling mediates stress-related alcohol preference in mice. PLoS One 10:e0122504
Wamsteeker Cusulin JI, Fuzesi T, Watts AG, Bains JS (2013) Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-Cre mutant mice. PLoS One 8:e64943
Wang XY, Guo YQ, Zhang WJ, Shao XX, Liu YL, Xu ZG, Guo ZY (2014) The electrostatic interactions of relaxin-3 with receptor RXFP4 and the influence of its B-chain C-terminal conformation. FEBS J 281:2927–2936
Watanabe Y, Miyamoto Y, Matsuda T, Tanaka M (2011) Relaxin-3/INSL7 regulates the stress-response system in the rat hypothalamus. J Mol Neurosci 43:169–174
van der Westhuizen ET, Halls ML, Samuel CS, Bathgate RAD, Unemori EN, Sutton SW, Summers RJ (2008) Relaxin family peptide receptors - from orphans to therapeutic targets. Drug Discov Today 13:640–651
Yan Y, Wang YL, Su Z, Zhang Y, Guo SX, Liu AJ, Wang CH, Sun FJ, Yang J (2014) Effect of oxytocin on the behavioral activity in the behavioral despair depression rat model. Neuropeptides 48:83–89
Yang J, Pan YJ, Yin ZK, Hai GF, Lu L, Zhao Y, Wang DX, Wang H, Wang G (2012) Effect of arginine vasopressin on the behavioral activity in the behavior despair depression rat model. Neuropeptides 46:141–149
Yoshikawa T, Nakajima Y, Yamada Y, Enoki R, Watanabe K, Yamazaki M, Sakimura K, Honma S, Honma K (2015) Spatiotemporal profiles of arginine vasopressin transcription in cultured suprachiasmatic nucleus. Eur J Neurosci 42:2678–2689
Young WS 3rd, Iacangelo A, Luo XZ, King C, Duncan K, Ginns EI (1999) Transgenic expression of green fluorescent protein in mouse oxytocin neurones. J Neuroendocrinol 11:935–939
Zee PC, Manthena P (2007) The brain’s master circadian clock: implications and opportunities for therapy of sleep disorders. Sleep Med Rev 11:59–70
Zhang L, Hernandez VS, Liu B, Medina MP, Nava-Kopp AT, Irles C, Morales M (2012) Hypothalamic vasopressin system regulation by maternal separation: its impact on anxiety in rats. Neuroscience 215:135–148
Zhang C, Chua BE, Yang A, Shabanpoor F, Hossain MA, Wade JD, Rosengren KJ, Smith CM, Gundlach AL (2015) Central relaxin-3 receptor (RXFP3) activation reduces elevated, but not basal, anxiety-like behaviour in C57BL/6J mice. Behav Brain Res 292:125–132
Acknowledgements
The authors thank Berenice Chua and Mouna Haidar for technical assistance, Dr Mohammad Akhter Hossain (The Florey Institute of Neuroscience and Mental Health) for providing RXFP3-A2 peptide, and Prof Paul Sawchenko and Dr Jean Rivier (Salk Institute, San Diego, USA) for supplying the rabbit CRH antiserum originally prepared in the Wylie Vale Laboratory, which was used in these studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Experiments were conducted with approval from The Florey Institute of Neuroscience and Mental Health Animal Ethics Committee, in compliance with guidelines of the National Health and Medical Research Council of Australia, and from the University of Calgary Animal Care and Use Committee in compliance with guidelines established by the Canadian Council on Animal Care.
Financial disclosure
This research was supported by a National Health and Medical Research Council of Australia Research Fellowship and Project Grant (1106330, 1024885, ALG), a Brain & Behavior Research Foundation (USA) NARSAD Independent Investigator Award (ALG); by the Canadian Institutes of Health Research and Brain Canada (JSB); and by the Victorian Government Operational Infrastructure Support Programme. CZ was the recipient of a Bethlehem Griffiths Research Foundation Postgraduate Scholarship and a Rebecca Hotchkiss International Scholar Exchange Travelling Fellowship.
Additional information
C. M. Smith, J. S. Bains and A. L. Gundlach jointly supervised this research.
Electronic supplementary material
.
Fig. S1
Comparative distribution of RXFP3 mRNA with that of oxytocin and arginine vasopressin mRNAs in mouse PVN determined by in situ hybridisation histochemistry (adapted from the Allen Brain Institute Atlas of Brain Gene Expression). (A) Schematic image of the anterior PVN (coronal level ~Bregma -0.70 mm). (B) RXFP3 mRNA is present in neurons located in a region of PVN that is similar to that occupied by neurons expressing oxytocin mRNA (C) and arginine vasopressin mRNA (D), suggesting a relationship between RXFP3 signalling and modulation of these neurohormone neurons. Abbreviations 3V, third ventricle; f, fornix; OT, oxytocin; AVP, arginine vasopressin. Scale bar, 300 μm. (PDF 259 kb)
.
Fig. S2
Comparative distribution of RXFP3 mRNA and oxytocin and arginine vasopressin mRNAs in mouse supraoptic nucleus (SON), determined by in situ hybridisation histochemistry (adapted from the Allen Brain Institute Atlas of Brain Gene Expression). (A) Schematic image of the SON (coronal level ~Bregma -0.82 mm). (B) RXFP3 mRNA is present in the magnocellular neurons within the supraoptic nucleus (SON), which express oxytocin mRNA (C) and arginine vasopressin mRNA (D), consistent with the presumed expression of RXFP3 mRNA by magnocellular neurons in the PVN (see Fig. S1), and further suggesting a relationship between RXFP3 signalling and these neurohormone neurons. In the supraoptic nucleus (SON), RXFP3 is expressed (B) in close proximity to oxytocin- (C) and vasopressin- (D) expressing neurons, further suggesting a relationship between RXFP3 signalling and these neuromodulatory peptides. Abbreviations: oc, optic chiasm. Scale bar, 100 μm. For further details of the distribution of RXFP1, RXFP3, CRH, OT and AVP mRNAs in the Allen Brain Institute Atlas of Brain Gene Expression, see <www.brain-map.org>. (PDF 196 kb)
Rights and permissions
About this article
Cite this article
Zhang, C., Baimoukhametova, D.V., Smith, C.M. et al. Relaxin-3/RXFP3 signalling in mouse hypothalamus: no effect of RXFP3 activation on corticosterone, despite reduced presynaptic excitatory input onto paraventricular CRH neurons in vitro. Psychopharmacology 234, 1725–1739 (2017). https://doi.org/10.1007/s00213-017-4575-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4575-z