Abstract
Rationale
The benzodiazepine lorazepam enhances the potential for inhibitory γ-aminobutyric acid (GABAA) synapses in the cortex to stabilize postsynaptic, excitatory activity by synchronizing discharge rates at frequencies of around 40 Hz. Treatment with lorazepam also affects contour integration processes, suggesting that GABAA-mediated synchronization plays a role in visuospatial organization. This conclusion is supported by other physiological studies that link visual feature integration with neuronal synchronization.
Objectives
One experiment was conducted to assess variations in dynamic figural priming as a result of lorazepam administration.
Methods
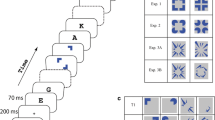
Observers were presented a modified version of a figural priming paradigm designed to investigate the effects of dynamic synchronization on visual feature integration. The priming paradigm consisted of premask crosses presented in a square arrangement within the same phase of a multiphase premask matrix oscillating at 40 Hz. Observers responded to a subsequently presented target square. The modification consisted of line elements presented at various distances relative to the unspecified extension of the lines making up the premask crosses. It was expected that priming effects would be enhanced for lines terminating close to the unspecified extension but only following administration of lorazepam.
Results
As anticipated, priming was enhanced substantially when the premask crosses flickered around static lines that terminated adjacent to the unspecified extension between the premask crosses. This effect was maximal following treatment with lorazepam.
Conclusions
This finding supports the idea that GABAA-enhanced inhibitory synchronization mediates continuity coding during early visual processing.



Similar content being viewed by others
Notes
Diazepam was chosen as a control for lorazepam in this experiment because although both drugs have equisedative effects, as measured subjectively and objectively, and equivalent effects on explicit memory (see, e.g., Legrand et al. 1995; Sellal et al. 1992; Vidailhet et al. 1994), they have been shown to differ in their effects on visual perception. In particular, lorazepam, but not diazepam, has been shown to specifically facilitate the detection of a discontinuity between collinear contours (Beckers et al. 2001; Giersch 1999, 2001), resulting in the impaired integration of local visual information into global configurations (Giersch et al. 1997; Giersch and Lorenceau 1999). Diazepam and lorazepam have also been shown to differ with respect to the identification of fragmented pictures (Legrand et al. 1995; Vidailhet et al. 1994; Wagemans et al. 1998), perceptual priming effects (Legrand et al. 1995; Vidailhet et al. 1994; Sellal et al. 1992), the detection of discontinuities in lines (Beckers et al. 2001), and in their effects on masking and vernier offset detection (Giersch and Herzog 2004). The reason why both drugs differ in these effects has still to be determined. On the one hand, there is evidence to suggest that diazepam and lorazepam may affect different subtypes of GABAA receptors (Mohler et al. 2002), but other more parsimonious explanations should also be considered, in particular the different influence of the two drugs on the dynamics of neural activity: while diazepam lowers the firing frequency of individual neurons in a similar fashion to lorazepam unlike lorazepam, it appears to have little influence on the generation of synchrony (Faulkner et al. 1998; Whittington et al. 1996). This characteristic tends to suggest that perceptual performance may be affected by lorazepam as a direct result of its effects upon the patterns of neural synchronization that have been found to emerge between neurons in the dynamic binding of visual features (i.e., Gray et al. 1989; Eckhorn et al. 1988; for review, see Singer 1999).
References
Beckers T, Wagemans J, Boucart M, Giersch A (2001) Different effects of lorazepam and diazepam on perceptual integration. Vision Res 41:2297–2303
Bell RA (1970) Application note 115. Principles of cathode-ray tubes, phosphors, and high-speed oscillography. Hewlett Packard Company/Colorado Springs Division, 1900 Garden of the Gods Road, Colorado Springs, Colorado, USA
Bond A, Lader M (1974). The use of analogue scales in rating subjective feelings. Br J Med Psychol 47:211–218
Boucart M, Delord S, Giersch A (1994) The computation of contour information in complex objects. Perception 23:399–409
Box GEP, Cox DR (1964) An analysis of transformations (with discussion). J R Stat Soc Ser B 26:211–246
Box GEP, Cox DR (1982) An analysis of transformations revisited, rebutted. J Am Stat Assoc 77:209–210
Dundee JW, McGowan WAW, Lilburn JA, McKay AC, Hegarty JE (1979) Comparison of the action of diazepam and lorazepam. Br J Anaesth 51:439–446
Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ (1988) Coherent oscillations: a mechanism for feature linking in the visual cortex. Biol Cybern 60:121–130
Elliott MA, Müller HJ (1998) Synchronous information presented in 40-Hz flicker enhances visual feature binding. Psychol Sci 9:277–283
Elliott MA, Müller HJ (2000) Evidence for a 40-Hz oscillatory short-term visual memory revealed by human reaction time measurements. J Exper Psychol Learn Mem Cogn 26:703–718
Elliott, MA, Müller HJ (2001) Effects of stimulus synchrony on mechanisms of perceptual organization. Vis Cogn 8:655–677
Elliott MA, Becker C, Boucart M, Müller HJ (2000) Enhanced GABAA inhibition enhances synchrony coding in human perception. NeuroReport 11:3403–3407
Faulkner HJ, Traub RD, Whittington MA (1998) Disruption of synchronous gamma oscillations in the rat hippocampal slice: a common mechanism of anaesthetic drug action. Br J Pharmacol 125:483–492
Finley G (1985) A high-speed point plotter for vision research. Technical note. Vision Res 25:1993–1997
Giersch A (1999) A new pharmacological tool to investigate integration processes. Vis Cogn 6:267–297
Giersch A (2001) The effects of lorazepam on visual integration processes: how useful for neuroscientists? Vis Cogn 8:549–563
Giersch A, Caparos S (2005) Focused attention is not enough to activate discontinuities in lines, but scrutiny is. Conscious Cogn 14:613–632
Giersch A, Herzog M (2004) Lorazepam strongly prolongs visual information processing. Neuropsychopharmacology 29:1386–1394
Giersch A, Lorenceau J (1999) Effects of a benzodiazepine, lorazepam, on motion integration and segmentation: an effect on the processing of line-ends? Vision Res 39:2017–2025
Gilbert CD, Wiesel TN (1989) Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci 9:2432–2442
Giersch A, Boucart M, Danion J-M, Vidailhet P, Legrand F (1995) Effects of lorazepam on perceptual integration of visual forms in healthy volunteers. Psychopharmacology 119:102–114
Giersch A, Boucart M, Speeg-Schatz C, Kauffmann-Muller F, Danion J-M (1996) lorazepam impairs perceptual integration of visual forms: a central effect. Psychopharmacology 126:260–270
Giersch A, Boucart M, Danion J-M (1997) Lorazepam, a benzodiazepine, induces atypical distractor effects with compound letters: a role for line ends in the processing of compound letters. Vis Cogn 4:337–372
Gove A, Grossberg S, Mingolla E (1995) Brightness perception, illusory contours, and corticogeniculate feedback. Vis Neurosci 12:1027–1052
Gray CM, König P, Engel AK, Singer W (1989) Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338:334–337
Huynh H, Feldt LS (1976) Estimation of the box correction for degrees of freedom from sample data in the randomized block and split block designs. J Educ Stat 1:69–82
Kennedy JM (1988) Line endings and subjective contours. Spat Vis 3:151–158
Kothary SP, Brown ACD, Pandit UA, Samra SK, Pandit SK (1981) Time course of antirecall effect of diazepam and lorazepam following oral administration. Anaesthesiology 55:641–644
Kovács I, Kozma P, Feher A, Benedek G (1999) Late maturation of visual spatial integration in humans. Proc Natl Acad Sci U S A 96:12204–12209
Legrand F, Vidailhet P, Danion JM, Grangé D, Giersch A, Van Der Linden M, Imbs JL (1995) Time course of the effects of diazepam and lorazepam on perceptual priming and explicit memory. Psychopharmacology 118:475–479
Leonard BE (1992) Fundamentals of psychopharmacology. Wiley, Chichester
Lesher GW, Mingolla E (1993) The role of edges and line-ends in illusory contour formation. Vision Res 33:2253–2270
Lorenceau J, Giersch A, Seriès P (2005) Dynamic competition between contour integration and contour segmentation probed with moving stimuli. Vision Res 45:103–116
Mohler H, Fritschy JM, Rudolph U (2002) A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300:2–8
Sellal F, Danion J-M, Kauffmann-Muller F, Grangé D, Imbs J-L, Van Der Linden M, Singer L (1992) Differential effects of diazepam and lorazepam on repetition priming in healthy volunteers. Psychopharmacology 108:371–379
Shimojo S, Silverman GH, Nakayama K (1989) Occlusion and the solution to the aperture problem for motion. Vision Res 29:619–626
Sillito AM (1977) Inhibitory processes underlying the directional specificity of simple complex and hyper complex cells in the cats visual cortex. J Physiol 271:699–720
Singer W (1999) Neuronal synchrony: a versatile code for the definition of relations? Neuron 24:49–65
Traub RD, Whittington MA, Stanford IM, Jefferys JGR (1996) A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature 383:621–624
Traub RD, Whittington MA, Buhl EH, Jefferys JGR, Faulkner HJ (1999) On the mechanism of the frequency shift in neuronal oscillations induced in rat hippocampal slices by tetanic stimulation. J Neurosci 19:1088–1105
Vidailhet P, Danion J-M, Kauffmann-Muller F, Grangé D, Giersch A, Van Der Linden M, Imbs J-L (1994) Lorazepam and diazepam effects on memory acquisition in priming tasks. Psychopharmacology 115:397–406
von der Heydt R, Peterhans E (1989) Mechanisms of contour perception in monkey visual cortex: 1. Lines of pattern discontinuities. J Neurosci 9:1731–1748
Wagemans J, Notebaert W, Boucart M (1998) Lorazepam but not diazepam impairs identification of pictures on the basis of specific contour fragments. Psychopharmacology 138:326–333
Westheimer G, Li W (1996) Classifying illusory contours by means of orientation discrimination. J Neurophysiol 75:523–528
Whittington MA, Traub RD, Jefferys JGR (1995) Synchronised oscillations in interneurons networks driven by metabolic glutamate activation. Nature 373:612–615
Whittington MA, Jefferys JGR, Traub RD (1996) Effects of intravenous anaesthetic agents on fast inhibitory oscillations in rat hippocampus in vitro. Br J Pharmacol 118:1977–1986
Whittington MA, Traub RD, Faulkner HJ, Stanford IM, Jefferys JGR (1997) Recurrent excitatory postsynaptic potentials induced by synchronised fast cortical oscillations. Proc Natl Acad Sci U S A 94:12198–12203
Acknowledgements
This research was supported by INSERM, the University Hospital of Strasbourg, and deutsche Forschungsgemeinschaft project grant EL 248/1-1. We would like to thank Dr. M. Welsch for medical examination of the healthy volunteers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elliott, M.A., Giersch, A. & Seifert, D. Some facilitatory effects of lorazepam on dynamic visual binding. Psychopharmacology 184, 229–238 (2006). https://doi.org/10.1007/s00213-005-0242-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0242-x