Abstract
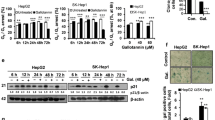
Apoptosis and autophagy have been shown to act cooperatively and antagonistically in self-elimination process. On the one side, apoptosis and autophagy can act as partners to induce cell death in a coordinated or cooperative manner; on the flip side, autophagy acts as an antagonist to block apoptotic cell death by promoting cell survival. Our previous research indicated that trillin could induce apoptosis of PLC/PRF/5 cells, but the effects of trillin on autophagy as well as its functional relationship to apoptosis have not been elucidated. Here, the running study aims to investigate the function and molecular mechanism of trillin on autophagy with hepatocellular carcinoma (HCC) cells. The objective of this study is to investigate the molecular mechanism of trillin on autophagy in HCC cells. Protein levels of autophagy markers beclin1, LC3B, and p62 were detected by western blotting. 6-Hydroxyflavone and stattic were used to test the role of trillin regulation of autophagy via serine threonine kinase (AKT)/extracellular-regulated protein kinases (ERK) 1/2/mammalian target of rapamycin (mTOR)/signal transducer and activator of transcription 3 (STAT3) signaling pathway. Flow cytometry was used to detect caspase 3 activity and apoptosis in PLC/PRF/5 cells treated with trillin for 24 h with or without rapamycin, stattic, and 6-hydroxyflavone. The protein level of autophagy marker beclin1 was decreased, whilst the protein level of p62 was significantly increased by trillin treatment, indicating trillin treatment led to inhibition of autophagy in HCC cells. Trillin treatment could reduce the protein levels of p-AKT and p-ERK1/2, but enhance the protein levels of mTOR and p-mTOR, suggesting that trillin could inhibit AKT/ERK rather than mTOR. The AKT/ERK activator 6-hydroxyflavone could reverse the loss of AKT and ERK1/2 phosphorylation induced by trillin, implying that trillin impairs autophagy through activated mTOR rather than AKT/ERK. STAT3 and p-STAT3 were significantly upregulated by the trillin treatment with an increase in dose from 0 to 50 μM, suggesting that autophagy inhibition is mediated by trillin via activation of STAT3 signaling. The STAT3 inhibitor stattic significantly reversed the increased STAT3 phosphorylation at tyrosine 705 induced by trillin. The mTOR signaling inhibitor rapamycin reversed the trillin-induced mTOR phosphorylation enhancement but exerted no effects on total mTOR levels, suggesting trillin treatment led to inhibition of autophagy in HCC cells through activating mTOR/STAT3 pathway. Furthermore, caspase 3 activities and the total rate of apoptosis were increased by trillin treatment, which was reversed by rapamycin, stattic, and 6-hydroxyflavone, proving that trillin promotes apoptosis via activation of mTOR/STAT3 signaling. Trillin induced autophagy inhibition and promoted apoptosis in PLC/PRF/5 cells via the activation of mTOR/STAT3 signaling. Trillin has the potential to be a viable therapeutic option for HCC treatment.







Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Al-Bari MAA, Xu P (2020) Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann N Y Acad Sci 1467:3–20. https://doi.org/10.1111/nyas.14305
Bronikowska J, Szliszka E, Kostrzewa-Susłow E, Jaworska D, Czuba ZP, Bednarski P, Król W (2017) Novel structurally related flavones augment cell death induced by rhsTRAIL. Int J Mol Sci 6:1211. https://doi.org/10.3390/ijms18061211
Bukowski K, Kciuk M, Kontek R (2020) Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 21:3233. https://doi.org/10.3390/ijms21093233
Dai W, Liu K, Li R, Cao Y, Shen M, Tao J, Liu H (2022) Trillin inhibits myoblast differentiation via increasing autophagy. Phytomedicine 99:153962. https://doi.org/10.1016/j.phymed.2022.153962
Egger ME, Huang JS, Yin W, McMasters KM, McNally LR (2013) Inhibition of autophagy with chloroquine is effective in melanoma. J Surg Res 1:274–81. https://doi.org/10.1016/j.jss.2013.04.055
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A (2009) Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 7:966–75. https://doi.org/10.1038/cdd.2009.33
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221:3–12. https://doi.org/10.1002/path.2697
Grabow WO, Puttergill DL, Bosch A (1992) Propagation of adenovirus types 40 and 41 in the PLC/PRF/5 primary liver carcinoma cell line. J Virol Methods 37(2):201–7. https://doi.org/10.1016/0166-0934(92)90047-h
Graf H, Jüngst C, Straub G, Dogan S, Hoffmann RT, Jakobs T et al (2008) Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion 78:34–38. https://doi.org/10.1159/000156702
Hsu YJ, Hsu SC, Huang SM, Lee HS, Lin SH, Tsai CS et al (2015) Hyperphosphatemia induces protective autophagy in endothelial cells through the inhibition of Akt/mTOR signaling. J Vasc Surg 62:210–221 e2. https://doi.org/10.1016/j.jvs.2014.02.040
Huang W, Zou K (2015) Cytotoxicity of the saponin TTB2 on Ewing sarcoma cells. Exp Ther Med 10:625–628. https://doi.org/10.3892/etm.2015.2544
Ikeda M, Morizane C, Ueno M, Okusaka T, Ishii H, Furuse J (2018) Chemotherapy for hepatocellular carcinoma: current status and future perspectives. Jpn J Clin Oncol 48:103–114. https://doi.org/10.1093/jjco/hyx180
Jiang P, Mizushima N (2015) LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 75: 13–8. https://doi.org/10.1016/j.ymeth.2014.11.021
Jiang T, Harder B, Rojo de la Vega M, Wong PK, Chapman E, Zhang DD (2015) p62 links autophagy and Nrf2 signaling. Free Radic Biol Med 88(Pt B):199–204.https://doi.org/10.1016/j.freeradbiomed.2015.06.014
Kim YC, Guan KL (2015) mTOR: a pharmacologic target for autophagy regulation. J Clin Invest 125(1):25–32. https://doi.org/10.1172/JCI73939
Kim JH, Yoon MS, Chen J (2009) Signal transducer and activator of transcription 3 (STAT3) mediates amino acid inhibition of insulin signaling through serine 727 phosphorylation. J Biol Chem 284:35425–35432. https://doi.org/10.1074/jbc.M109.051516
Kim DH et al (2003) GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell 11(4):895–904. https://doi.org/10.1016/s1097-2765(03)00114-x
Lai CH, Wu YW, Yeh SD, Lin YH, Tsai YH (2014) Effects of 6-hydroxyflavone on osteoblast differentiation in MC3T3-E1 Cells. Evid Based Complement Alternat Med 2014:924560. https://doi.org/10.1155/2014/924560
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132:27–42. https://doi.org/10.1016/j.cell.2007.12.018
Le Grazie M, Biagini MR, Tarocchi M, Polvani S, Galli A (2017) Chemotherapy for hepatocellular carcinoma: The present and the future. World J Hepatol 9:(21):907–920. https://doi.org/10.4254/wjh.v9.i21.907
Li Y, Sun Y, Fan L, Zhang F, Meng J, Han J et al (2014) Paris saponin VII inhibits growth of colorectal cancer cells through Ras signaling pathway. Biochem Pharmacol 88:150–157. https://doi.org/10.1016/j.bcp.2014.01.018
Li Y, Song Y, Li P, Li M, Wang H, Xu T et al (2020) Downregulation of RIG-I mediated by ITGB3/c-SRC/STAT3 signaling confers resistance to interferon-alpha-induced apoptosis in tumor-repopulating cells of melanoma. J Immunother Cancer 8:e000111. https://doi.org/10.1136/jitc-2019-000111
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang S et al (2017) Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis 8:e3015. https://doi.org/10.1038/cddis.2017.422
Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B (2017) The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull 7:339–348. https://doi.org/10.15171/apb.2017.041
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741. https://doi.org/10.1016/j.cell.2011.10.026
Ozakyol A (2017) Global epidemiology of hepatocellular carcinoma (HCC epidemiology). J Gastrointest Cancer 48:238–240. https://doi.org/10.1007/s12029-017-9959-0
Qian S, Tong S, Wu J, Tian L, Qi Z, Chen B et al (2020) Paris saponin VII extracted from Trillium tschonoskii induces autophagy and apoptosis in NSCLC cells. J Ethnopharmacol 248:112304. https://doi.org/10.1016/j.jep.2019.112304
Rangaraju S, Verrier JD, Madorsky I, Nicks J, Dunn WA Jr, Notterpek L (2010) Rapamycin activates autophagy and improves myelination in explant cultures from neuropathic mice. J Neurosci 30:11388–11397. https://doi.org/10.1523/JNEUROSCI.1356-10.2010
Schmitz KJ, Ademi C, Bertram S, Schmid KW, Baba HA (2016) Prognostic relevance of autophagy-related markers LC3, p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients with respect to KRAS mutational status. World J Surg Oncol 1:189. https://doi.org/10.1186/s12957-016-0946-x
Sishi BJ, Loos B, van Rooyen J, Engelbrecht AM (2013) Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochem Pharmacol 1:124–134. https://doi.org/10.1016/j.bcp.2012.10.005
Solomon VR, Lee H (2009) Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol 625:Chloroquine 0–233. https://doi.org/10.1016/j.ejphar.2009.06.063
Teng JF, Mei QB, Zhou XG, Tang Y, Xiong R, Qiu WQ et al (2020) Polyphyllin VI induces caspase-1-mediated pyroptosis via the induction of ROS / NF-kappaB / NLRP3 / GSDMD signal axis in non-small cell lung cancer. Cancers (Basel) 12:193. https://doi.org/10.3390/cancers12010193
Wang J, Zou K, Zhang Y, Liu C, Wu J, Zhou Y et al (2017) An 18-norspirostanol saponin with inhibitory action against COX-2 production from the underground part of Trillium tschonoskii. Chem Pharm Bull (Tokyo) 55:679–681. https://doi.org/10.1248/cpb.55.679
White E, Mehnert JM, Chan CS (2015) Autophagy, metabolism, and cancer. Clin Cancer Res 21:5037–5046. https://doi.org/10.1158/1078-0432.CCR-15-0490
Yokoyama T, Kondo Y, Kondo S (2007) Roles of mTOR and STAT3 in autophagy induced by telomere 3’ overhang-specific DNA oligonucleotides. Autophagy 3:496–498. https://doi.org/10.4161/auto.4602
You L, Wang Z, Li H, Shou J, Jing Z, Xie J et al (2015) The role of STAT3 in autophagy. Autophagy 5:729–739
Yu H, Lee H, Herrmann A, Buettner R, Jove R (2014) Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 14:736–746. https://doi.org/10.1038/nrc3818
Zhan G, Hu J, Xiao B, Wang X, Yang Z, Yang G et al (2020a) Trillin prevents proliferation and induces apoptosis through inhibiting STAT3 nuclear translocation in hepatoma carcinoma cells. Med Oncol 37:44. https://doi.org/10.1007/s12032-020-01369-7
Zhan S, Wang K, Xiang Q, Song Y, Li S, Liang H et al (2020b) lncRNA HOTAIR upregulates autophagy to promote apoptosis and senescence of nucleus pulposus cells. J Cell Physiol 235:2195–2208. https://doi.org/10.1002/jcp.29129
Zhang Q, Zhang C, He J, Guo Q, Hu D, Yang X et al (2015) STAT3 inhibitor stattic enhances radiosensitivity in esophageal squamous cell carcinoma. Tumour Biol 3:2135–2142. https://doi.org/10.1007/s13277-014-2823-y
Zhang P, Wang H, Chen Y, Lodhi AF, Sun C, Sun F et al (2020) DR5 related autophagy can promote apoptosis in gliomas after irradiation. Biochem Biophys Res Commun 522:910–916. https://doi.org/10.1016/j.bbrc.2019.11.161
Zou M, Hu C, You Q, Zhang A, Wang X, Guo Q (2015) Oroxylin A induces autophagy in human malignant glioma cells via the mTOR-STAT3-Notch signaling pathway. Mol Carcinog 54:1363–1375. https://doi.org/10.1002/mc.22212
Funding
This work was supported by the Grant of National Natural Science Foundation of China (Grant No. 81660718; 31800996), the Project of Hubei Provincial Key Laboratory of Occurrence and Intervention of Rheumatic Diseases (PT022101), and Key Projects of University Excellent Young Talents Support Plan (gxyqZD 2018061).
Author information
Authors and Affiliations
Contributions
Guangjie Zhan drafted the manuscript and made critical revision of the manuscript for important intellectual content. Tiantian Wei and Xiaoming Xie participated in the experiments and data collection. Jun Hu participated in the manuscript drafting. Hao Tang and Yating Cheng conducted the experiments. Huaifeng Liu and Huichen Xie did the statistical analysis of data. Guohua Yang and Shujing Li participated in the design of the study and the general supervision of the research group. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The authors of the manuscript entitled “Autophagy inhibition mediated by trillin promotes apoptosis in HCC cells via activation of mTOR/STAT3 signaling” declare that the requirement of the ethical approval is not applicable, since human and/or animal subjects were not conducted in this study.
Human and animal guidelines
The authors of the manuscript entitled “Autophagy inhibition mediated by trillin promotes apoptosis in HCC cells via activation of mTOR/STAT3 signaling” declare that the requirement of the Helsinki Declaration or relevant guidelines for animal experimentation is not applicable, since human and/or animal experiment was not conducted in this study.
Consent for publication
The authors of the manuscript entitled “Autophagy inhibition mediated by trillin promotes apoptosis in HCC cells via activation of mTOR/STAT3 signaling” confirm that written inform consent taken from the patients/volunteers/guardians of the children for this study is not applicable, since patients/volunteers/guardians of the children were not conducted in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Core tip
Trillium tschonoskii Maxim (TTM) have been previously reported to exert anti-tumor effects in lung cancer cells and colorectal cancer cell by inducing apoptosis. Total TTM steroid saponins can down-regulate the phosphorylation of AKT and ERK to induce apoptosis. Trillin is one particular component of total TTM steroid saponins and can markedly promote HCC cell apoptosis, inhibit invasion, and in turn block the development of HCC tumors. Here, the research investigated the possible effects of trillin and the signaling involved on autophagy in HCC cells, highlighting the suitability of trillin for the characterization of drug therapies for HCC.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhan, G., Wei, T., Xie, H. et al. Autophagy inhibition mediated by trillin promotes apoptosis in hepatocellular carcinoma cells via activation of mTOR/STAT3 signaling. Naunyn-Schmiedeberg's Arch Pharmacol 397, 1575–1587 (2024). https://doi.org/10.1007/s00210-023-02700-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02700-5