Abstract
We have previously shown that histamine (2-(1H-imidazol-4-yl)ethanamine) exerted concentration-dependent positive inotropic effects (PIE) or positive chronotropic effects (PCE) on isolated left and right atria, respectively, of transgenic (H2R-TG) mice that overexpress the human H2 histamine receptor (H2R) in the heart; however, the effects were not seen in their wild-type (WT) littermates. Amitriptyline, which is still a highly prescribed antidepressant drug, was reported to act as antagonist on H2Rs. Here, we wanted to determine whether the histamine effects in H2R-TG were antagonized by amitriptyline. Contractile studies were performed on isolated left and right atrial preparations, isolated perfused hearts from H2R-TG and WT mice and human atrial preparations. Amitriptyline shifted the concentration-dependent PIE of histamine (1 nM–10 μM) to higher concentrations (rightward shift) in left atrial preparations from H2R-TG. Similarly, in isolated perfused hearts from H2R-TG and WT mice, histamine increased the contractile parameters and the phosphorylation state of phospholamban (PLB) at serine 16 in the H2R-TG mice, but not in the WT mice. However, the increases in contractility and PLB phosphorylation were attenuated by the addition of amitriptyline in perfused hearts from H2R-TG. In isolated electrically stimulated human atria, the PIE of histamine that was applied in increasing concentrations from 1 nM to 10 μM was reduced by 10-μM amitriptyline. In summary, we present functional evidence that amitriptyline also acts as an antagonist of contractility at H2Rs in H2R-TG mouse hearts and in the human heart which might in part explain the side effects of amitriptyline.
Similar content being viewed by others
Introduction
Histamine is synthesized by cells, such as mast cells, in many organs of the mammalian body from histidine; histamine can also be ingested with food and is transported, in part, by thrombocytes via the coronary circulation to the heart (Jutel et al. 2009). Histamine can also be synthesized in the heart (Gergs et al. 2016; Grobe et al. 2016). Histamine has positive inotropic (PIE) and chronotropic effects (PCE), which were initially described in rabbits (Dale and Laidlaw 1910). These effects can be attributed to the stimulation of cardiac histamine receptors. Currently, the effects of histamine are thought to be mediated by four receptors: H1 receptor, H2 receptor, H3 receptor, and H4 receptor (Jutel et al. 2009). There are regional differences in the actions of histamine or in the utilization of histamine receptors in the mammalian heart. For example, H1 receptors mediate the PIE of histamine in rabbits probably because they activate phospholipase C (Hattori et al. 1988, 1990, 1991). In the left atrium of guinea pigs, the H1 receptor mediates the PIE of histamine, while the PCE in the guinea pig right atrium is mediated by the H2R; in the guinea pig ventricle, the PIE of histamine is mediated by the H2R (Zavecz and Levi 1978). H1 and H2 receptors have been detected in both human atrium and human ventricle using radioligand binding (Baumann et al. 1982, 1983, 1984), antibodies, and mRNA expression studies (Matsuda et al. 2004).
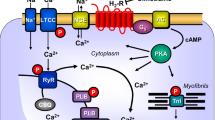
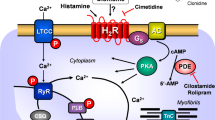
Cardiac H2Rs have been shown to mediate the PIE of exogenously applied histamine in isolated human cardiac preparations (Genovese et al. 1988; Levi et al. 1981; Sanders et al. 1996; Zerkowski et al. 1993). The PIE in the human heart was accompanied by and, thus, may have been mediated by an increase in 3’,5’-cyclic adenosine monophosphate (cAMP) content in human right atrial preparations (Sanders et al. 1996) and the opening of L-type calcium channels (Eckel et al. 1982, Fig. 1). Hence, the mode of action of the H2Rs in the human heart mimics the β-adrenoceptor system in the heart (Fig. 1). Stimulation of H2Rs generates cAMP in the heart, which leads to phospholamban (PLB) phosphorylation (H2R-TG, Gergs et al. 2019b).
Scheme of a cardiomyocyte: histamine can bind to the H2 histamine receptor in H2R-TG and human atrium; subsequently, the activity of adenylyl cyclase (AC) is augmented in the sarcolemma via stimulatory G-proteins (Gs); thereafter cAMP increases, and this activates the cAMP-dependent protein kinase (PKA). PKA increases cardiac force generation and relaxation by increasing the phosphorylation state (P) of the L-type Ca2+ channel (LTCC), phospholamban (PLB), and other regulatory proteins. Ca2+ initiates release of Ca2+ from the sarcoplasmic reticulum where it is usually bound to calsequestrin (CSQ) via ryanodine receptors (RYR) into the cytosol, where Ca2+ activates myofibrils and leads to increased inotropy. In the diastole, Ca2+ is taken up into the sarcoplasmic reticulum via a sarcoplasmic reticulum Ca2+ ATPase (SERCA), whose activity is higher when the phosphorylation state of PLB is elevated by PKA. The phosphorylation of proteins is reduced by protein phosphatases (PP). The H2R can be antagonized by amitriptyline; thus, PLB phosphorylation is not increased, force is not augmented, and relaxation is not hastened. Isoprenaline can stimulate likewise the β-adrenoceptor, which can also be antagonized by amitriptyline
Interestingly, some psychiatric drugs can act as antagonists on H2Rs, which has been shown using radioactive labelled ligands acting on H2Rs expressed in insect cells by a baculovirus system (Appl et al. 2012), human brain slices (Traiffort et al. 1992) and guinea pig hippocampus and cortex homogenates (Green and Maayani 1977). One study found that the most potent H2R antagonist (of psychiatric drugs studied) was amitriptyline with a pKi of 7.18 (Appl et al. 2012). Because amitriptyline is such a potent H2R antagonist, it was chosen for the present study. The therapeutic plasma concentration of amitriptyline has been reported to be 255–637 nM (Baumann et al. 2004). Hence, it was reasonable to assess whether the in vitro antagonism of human H2Rs by amitriptyline affected cardiac contractility.
Amitriptyline belongs to the class of tricyclic antidepressants (TCAs) and is commonly used as an antidepressant drug or for treatment of neuropathic pain and prevention of migraine. Although the antidepressant effect of amitriptyline is not completely understood, it is known to block the neuronal serotonin transporter and, in part, the neuronal noradrenaline transporter. Studies have shown that, in addition to the H2R, amitriptyline blocks many G protein-coupled receptors, including the muscarinic receptors, α-adrenoceptors, β-adrenoceptors, and H1 receptors, as well as the sodium and potassium ion channels (Appl et al. 2012; Bylund and Snyder 1976; Owens et al. 1997; Pancrazio et al. 1998; Punke and Friederich 2007; Sánchez and Hyttel 1999; Stanton et al. 1993). Amitriptyline is sometimes used as a sedative, but it is also increasing the action of analgesic drugs in patients with diabetic neuropathy (Punke and Friederich 2007). Regrettably, high doses of amitriptyline are used in suicide attempts (Henry 1997) and toxic plasma concentrations > 4 μM have been reported (Preskorn and Fast 1993). The cardiovascular side effects associated with amitriptyline include orthostatic hypotension, atrioventricular conduction delays, tachycardia, syncope, lengthening of the QT interval, and subsequent cardiac arrhythmias (Teschemacher et al. 1999). Overdoses of amitriptyline have also been reported to induce a Brugada-type ST elevation (Bolognesi et al. 1997; Brahmi et al. 2007). Further examples of cardiovascular side effects after amitriptyline overdosing are summarized in Table 1.
Green and Maayani (1977) found that amitriptyline inhibited histamine-stimulated adenylyl cyclase activity in the brain membranes of guinea pigs in a concentration-dependent manner and had in this regard a pA2 value of 7.23. Another study of an isolated spontaneously beating guinea pig right atrium found that amitriptyline failed to reduce the histamine-stimulated beating rate, although it antagonized the PIE of histamine in the papillary muscles and had a pKa value of 6.01 (Angus and Black 1980). These results suggested that amitriptyline had a region-specific effect on H2Rs.
The aim of the present study was to determine whether the inotropic and chronotropic effects of histamine in atrial preparations are sensitive to amitriptyline. The atrium from transgenic (H2R-TG) mice that were engineered to express a functional H2R on cardiomyocytes (Gergs et al. 2019a) and isolated electrically driven atrial strips from the human heart were used in this study. Preliminary reports of this project have been previously published in the form of abstracts (Binter et al. 2020; Neumann et al. 2019).
Materials and methods
Transgenic mice
H2R-TG mice with cardiac myocyte-specific overexpression of the human H2R were generated as described by Gergs et al. (2019a) and compared with their wild-type (WT) littermates as controls. The animals were handled and maintained according to the approved protocols (I8M9) of the Animal Welfare Committee of the University of Halle-Wittenberg, Germany.
Contractile studies in mice and human atrial preparations
In brief, the right or left atrial preparations were isolated from H2R-TG and WT mice and mounted in organ baths, as described by Gergs et al. (2013, 2017, 2019a) and Neumann et al. (2003). The contractile studies on the human atrium were performed as previously reported (Boknik et al. 2019). The human studies complied with the Declaration of Helsinki and followed the rules of the Ethics Committee of the University of Halle-Wittenberg (hm-bü 04.08.2005) and patients gave informed consent.
Western blotting
Western blotting, which involved homogenization, protein content measurements, electrophoresis and protein transfer, antibody incubations, and quantification, was performed following our established protocols (Boknik et al. 2018; Gergs et al. 2009, 2019a, b). For the electrophoresis, Novex™ 4–12% Tris-Glycine Plus Midi Protein gels (Invitrogen, Thermo Fisher Scientific, USA) were run for approximately 70 min at 120 V in the NuPAGE MES SDS Running Buffer (Life Technologies, USA) using the Bio-Rad system (Bio-Rad Laboratories, USA). The proteins were then transferred to a nitrocellulose blotting membrane (Amersham™ Protran, GE Healthcare, USA) at 2 A for 2 hours and at 4 °C. The primary antibodies for PLB (Ser16, Badrilla, UK) were incubated at 4 °C overnight. To visualize the phosphorylation of the analyzed proteins, ECF staining (ECF Substrate for Western Blotting, Amersham, GE Healthcare, USA) and a Typhoon 9410 Scanner (GE Healthcare, USA) were used. Quantification was performed using ImageQuant TL image analysis software (GE Healthcare, USA), as described by Boknik et al. (2018). It is typical for phospholamban that if the homogenate of the heart is kept at room temperature, it runs as a pentameric holoprotein of about 27 kDa. In contrast, upon brief elevation in the temperature (“boiling”), it runs as a monomer at less than 10 kDa (Wegener and Jones 1984). This peculiar physicochemical property can be used to identify phospholamban by Western blotting. In other words, the bands which we depicted here as phospholamban are converted to a smaller molecular weight band upon boiling and are still recognized by the phospholamban antibody. This is depicted in a control experiment in the supplementary Fig. 1 (compare also, e.g., Fig. 1, in Neumann et al. 1994).
Data analysis
The data were evaluated using the method previously reported by our group (Gergs et al. 2013, 2017, 2019b).
Drugs and materials
Amitriptyline was purchased from Sigma-Aldrich (Deisenhofen, Germany). The source of the other drugs was previously reported (Gergs et al. 2013, 2017, 2019b).
Results
Left and right atria of mice were prepared and after equilibration the following experimental procedure was conducted: First, a concentration response curve for histamine (1 nM–10 μM) was performed; thereafter, histamine was washed out, amitriptyline (1, 3 or 10 μM) was added, and a second concentration response curve for histamine was constructed. Finally, the β-adrenoceptor agonist isoprenaline (1 μM) was added to test the viability of the preparations (Fig. 2). The last step was necessary especially for WT preparations because histamine did not exert a PIE or PCE in the WT mice (Gergs et al. 2019b). Even though WT controls were performed, data for WT are not shown here because of the above mentioned lack of effects. A typical original recording for H2R-TG is shown in Fig. 2b and summarized in Fig. 2c. Our results showed that histamine exerted a PIE in isolated electrically stimulated (1 Hz) left atrial preparations from H2R-TG mice; the PIE was concentration and time dependent (–log EC50 = 7.4). To demonstrate that the histamine effects are mediated via the H2R in H2R-TG, an original recording of a concentration response curve shift for histamine by the H2R antagonist famotidine is presented (Fig. 2a). Amitriptyline shifted the concentration response curves for histamine to a higher concentration (Fig. 2c); the relationship between the amitriptyline and the histamine was concentration dependent. In the presence of 10-μM amitriptyline, the pEC50 value increased to 6.2, which was significantly higher than the pEC50 value without amitriptyline.
a Original recording of the force of contraction (FOC) in left atrium from transgenic mice that overexpress the H2 receptor (H2R-TG). First, a concentration response curve for histamine is shown; thereafter histamine was washed out, 10-μM amitriptyline was added, and a second concentration response curve for histamine was constructed. Finally, the β-adrenoceptor agonist isoprenaline was added. b Effect of histamine alone (open circles) or in the additional presence of 1-μM amitriptyline (closed circles) or 10-μM amitriptyline (red circles) on the FOC in isolated electrically driven (1 Hz) left atrium of H2R-TG. Ordinate: increase in force of contraction in relations to the maximum effect of histamine (=100%). Abscissa: logarithm of histamine concentration. ★indicates first significant difference (P < 0.05) vs. Ctr (= pre-drug value); #p < 0.05 versus control w/o amitriptyline
Moreover, in the left atrial preparations from H2R-TG mice, histamine increased the maximum rate of tension development in a concentration-dependent fashion (Fig. 3). The maximum and minimum rate of tension development was not affected by 1-μM amitriptyline, but in the presence of 3-μM amitriptyline, the pEC50 value was reduced from the control value of 7.51 to 7.21 (p < 0.05) (Fig. 3). Similarly, histamine tentatively increased the minimum rate of tension development in the left atrial preparations in a concentration-dependent fashion with a pEC50 value of 7.42 which was reduced to 7.28 (not significant) in the presence of 3-μM amitriptyline (Fig. 3). In addition, the effect of histamine on the maximum rate of tension development amounted to a pEC50 value of 7.18 which was changed to 6.44 (p < 0.05) in the presence of 10-μM amitriptyline (Fig. 3). Similarly, histamine increased the minimum rate of tension development with a pEC50 value of 7.19 which was reduced to 6.55 (p < 0.05) in the presence of 10-μM amitriptyline (Fig. 3).
Left side (a, c, e): effect of histamine alone (open circles) or in the additional presence of 1-μM (a), 3-μM (c), or 10-μM (e) amitriptyline (closed circles) on the maximum rate of force development in isolated electrically driven (1 Hz) left atrium of H2 histamine receptor overexpressing mice (H2R-TG). Ordinate in % of maximum change of force development (ΔdF/dtmax). Ctr = basal contraction before drug addition. Right side (b, d, f): effect of histamine alone (open circles) or in the additional presence of 1-μM (b), 3-μM (d), or 10-μM (f) amitriptyline (closed circles) on the minimum rate of force development in isolated electrically driven (1 Hz) left atrium of H2R-TG mice. Ordinate in % of minimum change of force development (ΔdF/dtmin). Ctr = basal contraction before drug addition. Abscissae: logarithm of histamine concentration. ★indicates first significant difference (P < 0.05) vs. Ctr; #p < 0.05 versus control w/o amitriptyline
Histamine shortened the time to peak tension (Tr) and amounted a pEC50 value of 6.83 which was reduced to 6.02 (p < 0.05) in the presence of 10-μM amitriptyline (Fig. 4). In addition, histamine accelerated the time of relaxation (Tf); likewise, this curve was shifted to higher concentrations of histamine in the presence of 10-μM amitriptyline (Fig. 4).
a Effect of histamine alone (open circles) or in the additional presence of 10-μM amitriptyline (closed circles) on the change of shortening in time to peak tension (Tr) in isolated electrically driven (1 Hz) left atrium of H2 histamine receptor overexpressing mice (H2R-TG). Ordinate: change in Tr in milliseconds (ms). Ctr = basal Tr before drug addition. b Effect of histamine alone (open circles) or in the additional presence of 10-μM amitriptyline (closed circles) on the change of shortening in time of relaxation (Tf) in isolated electrically driven (1 Hz) left atrium of H2R-TG mice. Ordinate: change in Tf in milliseconds (ms). Ctr = basal Tf before drug addition. Abscissae: logarithm of histamine concentration. ★indicates first significant difference (P < 0.05) vs. Ctr; #p < 0.05 versus control w/o amitriptyline
Histamine increased the beating rate in the right atrial preparations from H2R-TG mice (Fig. 5). The positive chronotropic effect of histamine amounted to pEC50 values 7.39 and shifted to 6.67 in the presence of 1-μM amitriptyline and from 7.24 to 6.36 (p < 0.05) with 3-μM amitriptyline (Fig. 5). We could not study the effects of 10-μM amitriptyline in the right atrial preparations because it consistently caused arrhythmias after application (data not shown).
Effect of histamine alone (open circles) or in the presence of 1-μM (closed circles) or 3-μM (red circles) amitriptyline in isolated spontaneously beating right atrium of H2R-TG. Ordinate: beating rate in beats per minute. Abscissae: logarithm of histamine concentration. ★indicates first significant difference (P < 0.05) vs. Ctr (= pre-drug value); #p < 0.05 versus control w/o amitriptyline
The previous data were obtained for atrial preparations from H2R-TG mice. For comparison, we studied the ventricular function in isolated spontaneously beating mouse hearts (Langendorff preparation). We found that 1-μM histamine exerted pronounced effects on the force of contraction in H2R-TG but not in WT hearts. However, this effect was nullified in the presence of 10-μM amitriptyline (data not shown). At the end of the contraction experiment, 5 min after addition of histamine, hearts were freeze clamped in liquid nitrogen and subsequently we determined whether the contractile changes in the perfused mouse hearts were accompanied by, and possibly caused by, biochemical alterations (compare Fig. 1). We noted that histamine could increase the phosphorylation state of phospholamban (PLB) at serine 16 (Fig. 6, supplementary Fig. 1). This effect was attenuated by additionally applied amitriptyline (Fig. 6, supplementary Fig. 1).
Western blot analysis of phospholamban (PLB) phosphorylation at serine 16 in Langendorff hearts from H2R-TG and WT mice perfused with histamine (1 μM) alone or in the combined presence with amitriptyline (10 μM). Calsequestrin (CSQ) was used as loading control. Ordinate: ratio of serine 16 phosphorylation of PLB and CSQ. *p < 0.05 vs indicated group. The numbers in the bars indicate the numbers of experiments. More details are shown in supplementary Fig. 1.
We also studied whether these contractile effects could also occur in the human heart. We found that 10-μM amitriptyline shifted the concentration response curve for the force of contraction of histamine in electrically stimulated human right atrial trabeculae carneae to higher concentrations (Fig. 7).
Effect of histamine alone (control, open circles) or in the additional presence of 10-μM amitriptyline (closed circles) on the force of contraction (FOC) in isolated electrically driven (1 Hz) human atrial preparations. Six preparations from four patients were used. ★p < 0.05 vs. Ctr (= pre-drug value); #p < 0.05 versus control w/o amitriptyline
Discussion
Right atria
In previous studies, we showed that histamine will elicit a PCE in isolated spontaneously beating right atrial preparations from H2R-TG mice (Gergs et al. 2019b, 2020). In the present study, we noted that amitriptyline had a concentration-dependent negative chronotropic effect, which might have resulted from its known antagonism of β-adrenoceptors. However, in right atrial preparations from H2R-TG mice, amitriptyline was able to shift the histamine concentration response curves to the right, which is consistent with the results from an antagonism of human H2Rs in the sinus node of the H2R-TG mice. Others before us reported that amitriptyline could attenuate the PCE of histamine in right atrial preparations of guinea pig and rabbit (Hughes and Coret 1974).
We noted another result in the right atria that may merit a mention. The isolated hearts from H2R-TG and WT mice showed a pronounced NIE from amitriptyline and several hearts exhibited transient arrhythmias. These results agree with the known propensity of amitriptyline to cause arrhythmias. However, amitriptyline has not yet been studied in mouse hearts. One study on guinea pigs reported that 10- and 32-μM amitriptyline reduced the spontaneous beating rate of right atrial preparations, an effect that was explained by the non-H2R-antagonizing properties of amitriptyline; however, amitriptyline failed to shift the histamine-induced PCE in these preparations (Angus and Black 1980). In contrast, 3.6- till 10-μM amitriptyline reduced the PCE of histamine in isolated rabbit and guinea pig atrial preparations hearts and amitriptyline exerted a negative chronotropic effect given alone (Hughes and Coret 1974). We would argue that we present the first evidence that amitriptyline antagonizes human H2Rs in the right atrium.
Left atria
We noticed that amitriptyline caused a small NIE in left atrial preparations from H2R-TG mice. This could be due to the blockade of sodium channels that has been reported for amitriptyline (Dick et al. 2007). Impairment of sodium entry would be expected to activate the sodium–calcium exchanger, which would cause sodium to be pumped into the cell in exchange for calcium. This is consistent with a NIE, which is the reason why class I antiarrhythmic agents are contraindicated in patients with systolic heart failure. A patch-clamp experiment showed that amitriptyline also blocked L-type calcium channels with an IC50 value of 23.2 μM, which would also in part explain the NIE of amitriptyline (Zahradník et al. 2008). It is clear that, in the left atrial preparations, histamine caused the contractile parameters, including force, the first derivative of force, time to peak tension, and time of relaxation, to shorten. This is consistent with our previously published work (Gergs et al. 2019b, 2020). Moreover, in our previous studies, we demonstrated that the effects of histamine we detect in H2R-TG are really due to H2R occupation. There, we could show that the positive inotropic effects of histamine in atrial preparations from H2R-TG are antagonized by the H2R antagonist cimetidine (1, 3, 10 μM: Gergs et al. 2019b). These data are in line with the famotidine effect presented here. The novel finding was that amitriptyline attenuated the contractile effects in a concentration-dependent way.
Another unexpected finding was that the PIE of isoprenaline, a β-adrenoceptor agonist, was also attenuated by amitriptyline. At the end of the experiment, we stimulated the samples from the WT and H2R-TG mice with isoprenaline to ascertain that the samples from the WT mice were responsive to β-adrenergic stimulation and, thus, were a valid control. In this experiment, we needed at least 100-μM isoprenaline to detect a PIE in the atria of the WT and H2R-TG mice. This result was consistent with receptor binding data that showed that amitriptyline had a β-adrenoceptor-antagonizing effect in the brain (Richardson and Hertz 1983; Sánchez and Hyttel 1999). The clinical relevance of this finding is unknown and speculative but might be determined by clinical groups in the future.
Langendorff hearts
As mentioned in the “Introduction” section, there are regional differences in the density and coupling of H2Rs in the heart. We have previously reported that in the H2R-TG mouse model, the PIE and PCE of histamine are noticeable in Langendorff hearts and are accompanied by PLB phosphorylation (Gergs et al. 2019b). Likewise, positive inotropic ventricular effects of histamine in Langendorff hearts were antagonized by cimetidine (Fig. 4d in Gergs et al. 2019b). One interpretation of this result was that it showed that histamine used the cAMP–PKA–PLB pathway in H2R-TG. In the present study, we found that amitriptyline not only attenuated the PIE of histamine but also the PLB phosphorylation due to histamine, which indicated that amitriptyline can also antagonize functional H2Rs in the mammalian ventricle. These findings confirmed and extended previous data in guinea pig ventricle (Angus and Black 1980). For example, amitriptyline was able to shift the histamine-induced PIE to the right because of its competitive antagonistic effect on the ventricular H2Rs in guinea pigs (Angus and Black 1980). However, other studies showed that the guinea pig papillary muscles contain H2 receptors and H1 receptors that can elicit an NIE (Zavecz and Levi 1978). Therefore, our data are more unambiguous than data in guinea pig ventricles because there are no functional H1 receptors in H2R-TG mice (Gergs et al. 2019b) in contrast to guinea pig ventricles (Zavecz and Levi 1978).
Human atria
We predicted that amitriptyline would also antagonize endogenous human cardiac H2Rs. Therefore, we performed contraction experiments on human atrial samples. Previous studies have repeatedly shown that histamine elicits a PIE in an isolated human atrium (see the “Introduction” section). In this study, we found that the PIE of histamine in electrical stimulated right atrial trabeculae carneae could be attenuated by amitriptyline. We also noted that high concentrations of isoprenaline, such as 100-μM isoprenaline, were needed to elicit a PIE in human atrial preparations when amitriptyline is present in the organ bath. This was consistent with our data on left atrial preparations of H2R-TG and with previous binding data (see the “Introduction” section). To the best of our knowledge, the antagonistic effect of amitriptyline in the human heart on histamine-induced effects had not been previously reported.
Limitations of the study
We were unable to study the effects of amitriptyline in human ventricular tissue because of a lack of available samples in our institution. We await such data with interest. There is also a question about the clinical relevance of our findings. Some contractile effects were noticeable with 1-μM amitriptyline, while other effects were only significant with 10-μM amitriptyline. The highest therapeutic level of amitriptyline given to psychiatric patients was 637 nM, which is not vastly different from 1 μM. Hence, small effects of amitriptyline on H2Rs might be apparent in properly treated patients. In a series of fatal intoxications associated with suicidal intentions in Finland, median concentrations of 12.6-μM amitriptyline were reported (Koski et al. 2005). Therefore, we argue that our findings are of toxicological relevance. Moreover, toxic plasma levels of amitriptyline might be reached even at normal dosages. Amitriptyline is metabolized mainly in the liver by cytochromes like CYP2D6, but also by CYP1A2, CYP2C9, CYP2C19, CYP3A4, and CYP3A5 (Samer et al. 2013). In a fatal intoxication with loss of functional CYP2D6 gene, a blood concentration of 60 mg/l (216 μM) was reported (Koski et al. 2006) and drugs that impair the degradation of amitriptyline have been reported to lead to intoxication (Forget et al. 2008). The data on plasma concentrations of amitriptyline and accompanying signs of intoxication are combined in Table 1 for better reference.
In summary, for the first time, we showed that amitriptyline can antagonize the contractile effects from the stimulation of a human cardiac H2R. We showed these effects in a H2R-TG mouse model, as well as in human cardiac atrium.
References
Angus JA, Black JW (1980) Pharmacological assay of cardiac H2-receptor blockade by amitriptyline and lysergic acid diethylamide. Circ Res 46(6 Pt 2):I64–I69
Appl H, Holzammer T, Dove S, Haen E, Strasser A, Seifert R (2012) Interactions of recombinant human histamine H1R, H2R, H3R, and H4R receptors with 34 antidepressants and antipsychotics. Naunyn-Schmiedebergs Arch Pharmacol 385(2):145–170
Baumann G, Felix SB, Riess G, Loher U, Ludwig L, Blömer H (1982) Effective stimulation of cardiac contractility and myocardial metabolism by impromidine and dimaprit--two new H2-agonistic compounds--in the surviving, catecholamine-insensitive myocardium after coronary occlusion. J Cardiovasc Pharmacol 4(4):542–553. https://doi.org/10.1097/00005344-198207000-00004
Baumann G, Mercader D, Busch U, Felix SB, Loher U, Ludwig L, Sebening H, Heidecke CD, Hagl S, Sebening F, Blömer H (1983) Effects of the H2-receptor agonist impromidine in human myocardium from patients with heart failure due to mitral and aortic valve disease. J Cardiovasc Pharmacol 5(4):618–625. https://doi.org/10.1097/00005344-198307000-00017
Baumann G, Permanetter B, Wirtzfeld A (1984) Possible value of H2-receptor agonists for treatment of catecholamine-insensitive congestive heart failure. Pharmacol Ther 24(2):165–177. https://doi.org/10.1016/0163-7258(84)90033-0
Baumann P, Hiemke C, Ulrich S, Eckermann G, Gaertner I, Gerlach M, Kuss HJ, Laux G, Müller-Oerlinghausen B, Rao ML, Riederer P, Zernig G (2004) The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry 37(6):243–265. https://doi.org/10.1055/s-2004-832687
Binter M, Gergs U, Neumann J (2020) Interaction of amitriptyline and histamine in the heart of H2 receptor transgenic mice. Naunyn-Schmiedeberg’s Arch Pharmacol 393(Suppl 1):S31 (abstract)
Boknik P, Drzewiecki K, Eskandar J, Gergs U, Grote-Wessels S, Fabritz L, Kirchhof P, Müller FU, Stümpel F, Schmitz W, Zimmermann N, Kirchhefer U, Neumann J (2018) Phenotyping of mice with heart specific overexpression of A2A-adenosine receptors: Evidence for cardioprotective effects of A2A-adenosine receptors. Front Pharmacol 9:13. https://doi.org/10.3389/fphar.2018.00013
Boknik P, Drzewiecki K, Eskandar J, Gergs U, Hofmann B, Treede H, Grote-Wessels S, Fabritz L, Kirchhof P, Fortmüller L, Müller FU, Schmitz W, Zimmermann N, Kirchhefer U, Neumann J (2019) Evidence for arrhythmogenic effects of A2A-adenosine receptors. Front Pharmacol 10:1051. https://doi.org/10.3389/fphar.2019.01051
Bolognesi R, Tsialtas D, Vasini P, Conti M, Manca C (1997) Abnormal ventricular repolarization mimicking myocardial infarction after heterocyclic antidepressant overdose. Am J Cardiol 79(2):242–245. https://doi.org/10.1016/s0002-9149(96)00727-8
Brahmi N, Thabet H, Kouraichi N, Driss I, Amamou M (2007) Brugada syndrome and other cardiovascular abnormalities related to tricyclic antidepressants and related drugs intoxication. Arch Mal Coeur Vaiss 100(1):28–33
Bylund DB, Snyder SH (1976) Beta adrenergic receptor binding in membrane preparations from mammalian brain. Mol Pharmacol 12(4):568–580
Caksen H, Akbayram S, Odabaş D, Ozbek H, Erol M, Akgün C, Tuncer O, Yilmaz C (2006) Acute amitriptyline intoxication: an analysis of 44 children. Hum Exp Toxicol 25(3):107–110. https://doi.org/10.1191/0960327106ht511oa
Dale HH, Laidlaw PP (1910) The physiological action of beta-iminazolylethylamine. J Physiol 41(5):318–344. https://doi.org/10.1113/jphysiol.1910.sp001406
Dick IE, Brochu RM, Purohit Y, Kaczorowski GJ, Martin WJ, Priest BT (2007) Sodium channel blockade may contribute to the analgesic efficacy of antidepressants. J Pain 8(4):315–324. https://doi.org/10.1016/j.jpain.2006.10.001
Druid H, Holmgren P (1997) A compilation of fatal and control concentrations of drugs in postmortem femoral blood. J Forensic Sci 42(1):79–87
Eckel L, Gristwood RW, Nawrath H, Owen DA, Satter P (1982) Inotropic and electrophysiological effects of histamine on human ventricular heart muscle. J Physiol 330:111–123. https://doi.org/10.1113/jphysiol.1982.sp014332
Forget P, le Polain de Waroux B, Wallemacq P, Gala JL (2008) Life-threatening dextromethorphan intoxication associated with interaction with amitriptyline in a poor CYP2D6 metabolizer: a single case re-exposure study. J Pain Symptom Manage 36(1):92–96. https://doi.org/10.1016/j.jpainsymman.2007.09.006
Genovese A, Gross SS, Sakuma I, Levi R (1988) Adenosine promotes histamine H1-mediated negative chronotropic and inotropic effects on human atrial myocardium. J Pharmacol Exp Ther 247(3):844–849
Gergs U, Neumann J, Simm A, Silber RE, Remmers FO, Läer S (2009) Phosphorylation of phospholamban and troponin I through 5-HT4-receptors in the isolated human atrium. Naunyn Schmiedebergs Arch Pharmacol 379(4):349–359. https://doi.org/10.1007/s00210-008-0371-y
Gergs U, Böckler A, Ebelt H, Hauptmann S, Keller N, Otto V, Pönicke K, Schmitz W, Neumann J (2013) Human 5-HT4-receptor stimulation in atria of transgenic mice. Naunyn Schmiedebergs Arch Pharmacol 386(5):357–367. https://doi.org/10.1007/s00210-013-0831-x
Gergs U, Grobe JM, Neumann J (2016) Diamine oxidase and monoamine oxidase can degrade histamine in the mammalian heart to an inotropically relevant extent. Inflamm Res 65(Suppl 1):S52 (abstract)
Gergs U, Fritsche J, Fabian S, Christ J, Neumann J (2017) Desensitization of the human 5-HT4 receptor in isolated atria of transgenic mice. Naunyn Schmiedebergs Arch Pharmacol 390(10):987–996. https://doi.org/10.1007/s00210-017-1403-2
Gergs U, Trapp T, Bushnaq H, Simm A, Silber RE, Neumann J (2019a) Age-dependent protein expression of serine-/threonine-phosphatases and their inhibitors in the human cardiac atrium. Adv Med 2019:2675972. https://doi.org/10.1155/2019/2675972
Gergs U, Bernhardt G, Buchwalow IB, Edler H, Fröba J, Keller M, Kirchhefer U, Köhler F, Mißlinger N, Wache H, Neumann J (2019b) Initial characterization of transgenic mice overexpressing human histamine H2 receptors. J Pharmacol Exp Ther 369(1):129–141. https://doi.org/10.1124/jpet.118.255711
Gergs U, Kirchhefer U, Bergmann F, Künstler B, Mißlinger N, Au B, Mahnkopf M, Wache H, Neumann J (2020) Characterization of stressed transgenic mice overexpressing H2-Histamine receptors in the heart. J Pharmacol Exp Ther 374(3):479–488. https://doi.org/10.1124/jpet.120.000063
Green JP, Maayani S (1977) Tricyclic antidepressant drugs block histamine H2 receptor in brain. Nature 269(5624):163–165. https://doi.org/10.1038/269163a0
Grobe J, Gergs U, Neumann J (2016) Functional studies on histamine metabolism in the mammalian heart. Naunyn-Schmiedeberg’s Arch Pharmacol 389(Suppl 1):S38 (abstract)
Hattori Y, Sakuma I, Kanno M (1988) Differential effects of histamine mediated by histamine H1- and H2-receptors on contractility, spontaneous rate and cyclic nucleotides in the rabbit heart. Eur J Pharmacol 153(2-3):221–229. https://doi.org/10.1016/0014-2999(88)90609-7
Hattori Y, Nakaya H, Endou M, Kanno M (1990) Inotropic, electrophysiological and biochemical responses to histamine in rabbit papillary muscles: evidence for coexistence of H1- and H2-receptors. J Pharmacol Exp Ther 253(1):250–256
Hattori Y, Gando S, Endou M, Kanno M (1991) Characterization of histamine receptors modulating inotropic and biochemical activities in rabbit left atria. Eur J Pharmacol 196(1):29–36. https://doi.org/10.1016/0014-2999(91)90405-f
Henry JA (1997) Epidemiology and relative toxicity of antidepressant drugs in overdose. Drug Saf 16:374–390
Hughes MJ, Coret IA (1974) Effects of tricyclic compounds on the histamine response of isolated atria. J Pharmacol Exp Ther 191(2):252–261
Jutel M, Akdis M, Akdis CA (2009) Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy 39(12):1786–1800. https://doi.org/10.1111/j.1365-2222.2009.03374.x
King LA (1982) Synergistic effect of benzodiazepines in fatal amitriptyline poisonings. Lancet 2(8305):982–983. https://doi.org/10.1016/s0140-6736(82)90177-5
Koski A, Vuori E, Ojanperä I (2005) Relation of postmortem blood alcohol and drug concentrations in fatal poisonings involving amitriptyline, propoxyphene and promazine. Hum Exp Toxicol 24(8):389–396. https://doi.org/10.1191/0960327105ht542oa
Koski A, Sistonen J, Ojanperä I, Gergov M, Vuori E, Sajantila A (2006) CYP2D6 and CYP2C19 genotypes and amitriptyline metabolite ratios in a series of medicolegal autopsies. Forensic Sci Int 158(2-3):177–183. https://doi.org/10.1016/j.forsciint.2005.05.032
Langou RA, Van Dyke C, Tahan SR, Cohen LS (1980) Cardiovascular manifestations of tricyclic antidepressant overdose. Am Heart J 100(4):458–464. https://doi.org/10.1016/0002-8703(80)90657-2
Levi R, Malm JR, Bowman FO, Rosen MR (1981) The arrhythmogenic actions of histamine on human atrial fibers. Circ Res 49(2):545–550. https://doi.org/10.1161/01.res.49.2.545
Matsuda N, Jesmin S, Takahashi Y, Hatta E, Kobayashi M, Matsuyama K, Kawakami N, Sakuma I, Gando S, Fukui H, Hattori Y, Levi R (2004) Histamine H1 and H2 receptor gene and protein levels are differentially expressed in the hearts of rodents and humans. J Pharmacol Exp Ther 309(2):786–795. https://doi.org/10.1124/jpet.103.063065
Neumann J, Boknik P, Bodor GS, Jones LR, Schmitz W, Scholz H (1994) Effects of adenosine receptor and muscarinic cholinergic receptor agonists on cardiac protein phosphorylation. Influence of pertussis toxin. J Pharmacol Exp Ther 269:1310–1318
Neumann J, Boknik P, Matherne GP, Lankford A, Schmitz W (2003) Pertussis toxin sensitive and insensitive effects of adenosine and carbachol in murine atria overexpressing A1–adenosine receptors. Br J Pharmacol 138(1):209–217. https://doi.org/10.1038/sj.bjp.070501
Neumann J, Schwarzer D, Binter M, Gergs U (2019) Interaction of H2-histamine and 5-HT4-serotonin receptors in the mammalian heart and effects of amitriptyline. Inflamm Res 68(Suppl 1):S45 (abstract)
Olgun H, Yildirim ZK, Karacan M, Ceviz N (2009) Clinical, electrocardiographic, and laboratory findings in children with amitriptyline intoxication. Pediatr Emerg Care 25(3):170–173. https://doi.org/10.1097/PEC.0b013e31819a8994
Owens MJ, Morgan WN, Plott SJ, Nemeroff CB (1997) Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther 283(3):1305–1322
Paksu S, Duran L, Altuntas M, Zengin H, Salis O, Ozsevik SN, Albayrak H, Murat N, Guzel A, Paksu MS (2014) Amitriptyline overdose in emergency department of university hospital: evaluation of 250 patients. Hum Exp Toxicol 33(9):980–990. https://doi.org/10.1177/0960327113520019
Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch C 3rd (1998) Inhibition of neuronal Na+ channels by antidepressant drugs. J Pharmacol Exp Ther 284(1):208–214
Preskorn SH, Fast GA (1993) Beyond signs and symptoms: the case against a mixed anxiety and depression category. J Clin Psychiatry 54(Suppl):24–32
Punke MA, Friederich P (2007) Amitriptyline is a potent blocker of human Kv1.1 and Kv7.2/7.3 channels. Anesth Analg 104(5):1256–1264. https://doi.org/10.1213/01.ane.0000260310.63117.a2
Richardson JS, Hertz L (1983) The effects of antidepressant drugs on adenylyl cyclase linked beta adrenergic binding sites on mouse astrocytes in primary cultures. Prog Neuropsychopharmacol Biol Psychiatry 7(4-6):675–680. https://doi.org/10.1016/0278-5846(83)90044-1
Samer CF, Lorenzini KI, Rollason V, Daali Y, Desmeules JA (2013) Applications of CYP450 testing in the clinical setting. Mol Diagn Ther 17(3):165–184. https://doi.org/10.1007/s40291-013-0028
Sánchez C, Hyttel J (1999) Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol 19(4):467–489. https://doi.org/10.1023/a:1006986824213
Sanders L, Lynham JA, Kaumann AJ (1996) Chronic beta 1-adrenoceptor blockade sensitises the H1 and H2 receptor systems in human atrium: rôle of cyclic nucleotides. Naunyn Schmiedebergs Arch Pharmacol 353(6):661–670. https://doi.org/10.1007/BF00167185
Scherf-Clavel M, Zebner J, Hommers L, Deckert J, Menke A, Unterecker S (2020) Nortriptyline serum concentration as a predictor for cardiac risk in amitriptyline-treated patients. Eur J Clin Pharmacol 76(1):73–80. https://doi.org/10.1007/s00228-019-02766-2
Stanton T, Bolden-Watson C, Cusack B, Richelson E (1993) Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics. Biochem Pharmacol 45(11):2352–2354. https://doi.org/10.1016/0006-2952(93)90211-e
Stead AH, Moffat AC (1983) A collection of therapeutic, toxic and fatal blood drug concentrations in man. Hum Toxicol 2(3):437–464. https://doi.org/10.1177/096032718300200301
Sunshine I, Baeumler J (1963) A fatal case of poisoning with amitriptyline. Nature 199:1103–1104. https://doi.org/10.1038/1991103a0
Teschemacher AG, Seward EP, Hancox JC, Witchel HJ (1999) Inhibition of the current of heterologously expressed HERG potassium channels by imipramine and amitriptyline. Br J Pharmacol 128(2):479–485. https://doi.org/10.1038/sj.bjp.0702800
Traiffort E, Pollard H, Moreau J, Ruat M, Schwartz JC, Martinez-Mir MI, Palacios JM (1992) Pharmacological characterization and autoradiographic localization of histamine H2 receptors in human brain identified with [125I]iodoaminopotentidine. J Neurochem 59(1):290–299. https://doi.org/10.1111/j.1471-4159.1992.tb08903.x
Wegener AD, Jones LR (1984) Phosphorylation-induced mobility shift in phospholamban in sodium dodecyl sulfate-polyacrylamide gels. Evidence for a protein structure consisting of multiple identical phosphorylatable subunits. J Biol Chem 259(3):1834–1841
Winek CL, Wahba WW, Winek CL Jr, Balzer TW (2001) Drug and chemical blood-level data 2001. Forensic Sci Int 122(2-3):107–123. https://doi.org/10.1016/s0379-0738(01)00483-2
Zahradník I, Minarovic I, Zahradníková A (2008) Inhibition of the cardiac L-type calcium channel current by antidepressant drugs. J Pharmacol Exp Ther 324(3):977–984. https://doi.org/10.1124/jpet.107.132456
Zavecz JH, Levi R (1978) Histamine-induced negative inotropism: mediation by H1-receptors. J Pharmacol Exp Ther 206(2):274–280
Zerkowski HR, Broede A, Kunde K, Hillemann S, Schäfer E, Vogelsang M, Michel MC, Brodde OE (1993) Comparison of the positive inotropic effects of serotonin, histamine, angiotensin II, endothelin and isoprenaline in the isolated human right atrium. Naunyn Schmiedebergs Arch Pharmacol 347(4):347–352. https://doi.org/10.1007/BF00165383
Acknowledgments
The work contains parts of the medical theses of MBB and CF. The technical assistance of S. Reber and P. Willmy is gratefully acknowledged.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JN and UK designed the research; MBB, CF, MM, and MLB performed the research; BH, UG, and JN analyzed the data; UG prepared the figures; and JN, UK, and BH wrote the paper. All authors read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 273 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neumann, J., Binter, M.B., Fehse, C. et al. Amitriptyline functionally antagonizes cardiac H2 histamine receptors in transgenic mice and human atria. Naunyn-Schmiedeberg's Arch Pharmacol 394, 1251–1262 (2021). https://doi.org/10.1007/s00210-021-02065-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-021-02065-7