Abstract
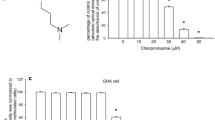
Haloperidol, a typical antipsychotic medication, has been shown to possess various biological effects in different brain models. However, the impact of haloperidol on Ca2+ signaling in astrocytes is elusive. This study explored the effect of haloperidol on cytosolic free Ca2+ levels ([Ca2+]i) and viability, and established these two connections in Gibco® Human Astrocytes (GHAs) and DI TNC1 rat astrocytes. Haloperidol (5–20 μM) caused [Ca2+]i rises in a concentration-dependent manner in GHAs but not in DI TNC1 cells. Furthermore, removal of extracellular Ca2+ reduced haloperidol’s effect by approximately 30% in GHAs. Haloperidol (20–40 μM) evoked concentration-dependent cytotoxicity in GHAs and DI TNC1 cells. However, chelating cytosolic Ca2+ with the Ca2+ chelator BAPTA/AM significantly reversed haloperidol’s cytotoxicity only in GHAs. In GHAs, haloperidol-induced Ca2+ entry was inhibited by store-operated Ca2+ modulators (2-APB and SKF96365) and the protein kinase C (PKC) inhibitor GF109203X. This Ca2+ entry induced by haloperidol was confirmed by Mn2+ entry-induced quench of fura-2 fluorescence. In Ca2+-free medium, treatment with the endoplasmic reticulum Ca2+ pump inhibitor 2,5-di-tert-butylhydroquinone (BHQ) abolished haloperidol-induced [Ca2+]i rises. Conversely, treatment with haloperidol inhibited 45% of BHQ-evoked [Ca2+]i rises. Moreover, haloperidol-induced Ca2+ release from the endoplasmic reticulum was abolished by inhibition of phospholipase C (PLC) by U73122. Together, in GHAs but not in DI TNC1 cells, haloperidol caused Ca2+-associated cell death, induced Ca2+ entry via PKC-sensitive store-operated Ca2+ channels, and evoked PLC-dependent Ca2+ release from the endoplasmic reticulum. The protective effect of Ca2+ chelating on haloperidol-induced cytotoxicity in human astrocytes was also demonstrated.







Similar content being viewed by others
References
Akamine T, Nishimura Y, Ito K, Uji Y, Yamamoto T (2002) Effects of haloperidol on K(+) currents in acutely isolated rat retinal ganglion cells. Invest Ophthalmol Vis Sci 43:1257–1261
Anderson CM, Bergher JP, Swanson RA (2004) ATP-induced ATP release from astrocytes. J Neurochem 88:246–256
Awouters FH, Lewi PJ (2007) Forty years of antipsychotic drug research—from haloperidol to paliperidone—with Dr. Paul Janssen. Arzneimittelforschung 57:625–632
Berridge MJ, Bootman MD, Lipp P (1998) Calcium-a life and death signal. Nature 395:645–648
Berridge MJ (2006) Calcium microdomains: organization and function. Cell Calcium 40:405–412
Berridge MJ (2013) Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion 7:2–13
Bootman MD, Berridge MJ, Roderick HL (2002) Calcium signalling: more messengers, more channels, more complexity. Curr Biol 12:R563–R565
Bynagari-Settipalli YS, Lakhani P, Jin J, Bhavaraju K, Rico MC, Kim S, Woulfe D, Kunapuli SP (2012) Protein kinase C isoform ε negatively regulates ADP-induced calcium mobilization and thromboxane generation in platelets. Arterioscler Thromb Vasc Biol 32:1211–1219
Clapham DE (2007) Calcium signaling. Cell 131:1047–1058
Ding S (2013) In vivo astrocytic Ca2+ signaling in health and brain disorders. Future Neurol 8:529–554
Fiacco TA, Agulhon C, McCarthy KD (2009) Sorting out astrocyte physiology from pharmacology. Annu Rev Pharmacol Toxicol 49:151–174
Florenzano F, Viscomi MT, Mercaldo V, Longone P, Bernardi G, Bagni C (2006) P2X2R purinergic receptor subunit mRNA and protein are expressed by all hypothalamic hypocretin/orexin neurons. J Comp Neurol 498:58–67
Giancotti FG (2014) Deregulation of cell signaling in cancer. FEBS Lett 588:2558–2570
Gottfried C, Valentim L, Salbego C, Karl J, Wofchuk ST, Rodnight R (1999) Regulation of protein phosphorylation in astrocyte cultures by external calcium ions: specific effects on the phosphorylation of glial fibrillary acidic protein (GFAP), vimentin and heat shock protein 27 (HSP27). Brain Res 833:142–149
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Harada M, Luo X, Murohara T, Yang B, Dobrev D, Nattel S (2014) MicroRNA regulation and cardiac calcium signaling: role in cardiac disease and therapeutic potential. Circ Res 114:689–705
Ishida H, Hoshiai K, Hoshiai M, Genka C, Hirota Y, Nakazawa H (1999) Haloperidol prolongs diastolic phase of Ca2+ transient in cardiac myocytes. Jpn J Physiol 49:479–484
Kamendulis LM, Jiang J, Zhang H, deFeijter-Rupp H, Trosko JE, Klaunig JE (1999) The effect of acrylonitrile on gap junctional intercellular communication in rat astrocytes. Cell Biol Toxicol 15:173–183
Kaufman RJ, Malhotra JD (2014) Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics. Biochim Biophys Acta 1843:2233–2239
Kim HS, Yumkham S, Choi JH, Kim EK, Kim YS, Ryu SH, Suh PG (2006) Haloperidol induces calcium ion influx via L-type calcium channels in hippocampal HN33 cells and renders the neurons more susceptible to oxidative stress. Mol Cells 22:51–57
Knol W, van Marum RJ, Jansen PA, Egberts TC, Schobben AF (2012) Parkinsonism in elderly users of haloperidol: associated with dose, plasma concentration, and duration of use. J Clin Psychopharmacol 32:688–693
Konopaske GT, Bolo NR, Basu AC, Renshaw PF, Coyle JT (2013) Time-dependent effects of haloperidol on glutamine and GABA homeostasis and astrocyte activity in the rat brain. Psychopharmacology 230:57–67
Li JH, Zhao ST, Wu CY, Cao X, Peng MR, Li SJ, Liu XA, Gao TM (2013) Store-operated Ca2+ channels blockers inhibit lipopolysaccharide induced astrocyte activation. Neurochem Res 38:2216–2226
Merritt JE, Jacob R, Hallam TJ (1989) Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem 264:1522–1527
Mitchell IJ, Cooper AC, Griffiths MR, Cooper AJ (2002) Acute administration of haloperidol induces apoptosis of neurones in the striatum and substantia nigra in the rat. Neuroscience 109:89–99
Noh JS, Kang HJ, Kim EY, Sohn S, Chung YK, Kim SU, Gwag BJ (2000) Haloperidol-induced neuronal apoptosis: role of p38 and c-Jun-NH(2)-terminal protein kinase. J Neurochem 75:2327–2334
Nordenberg J, Perlmutter I, Lavie G, Beery E, Uziel O, Morgenstern C, Fenig E, Weizman A (2005) Anti-proliferative activity of haloperidol in B16 mouse and human SK-MEL-28 melanoma cell lines. Int J Oncol 27:1097–1103
Novakova M, Sedlakova B, Sirova M, Fialova K, Krizanova O (2010) Haloperidol increases expression of the inositol 1,4,5-trisphosphate receptors in rat cardiac atria, but not in ventricles. Gen Physiol Biophys 29:381–389
Okubo Y, Iino M, Hirose K (2020) Store-operated Ca2+ entry-dependent Ca2+ refilling in the endoplasmic reticulum in astrocytes. Biochem Biophys Res Commun 522:1003–1008
Olivera JF, Pizarro G (2010) Two inhibitors of store operated Ca2+ entry suppress excitation contraction coupling in frog skeletal muscle. J Muscle Res Cell Motil 31:127–139
Palotás A, Penke B, Palotás M, Kenderessy AS, Kemény L, Kis E, Vincze G, Janka Z, Kálmán J (2004) Haloperidol attenuates beta-amyloid-induced calcium imbalance in human fibroblasts. Skin Pharmacol Physiol 17:195–199
Pillai A, Dhandapani KM, Pillai BA, Terry AV Jr, Mahadik SP (2008) Erythropoietin prevents haloperidol treatment-induced neuronal apoptosis through regulation of BDNF. Neuropsychopharmacology 33:1942–1951
Putney JW Jr (1986) A model for receptor-regulated calcium entry. Cell Calcium 7:1–12
Quincozes-Santos A, Bobermin LD, Tonial RP, Bambini-Junior V, Riesgo R, Gottfried C (2010) Effects of atypical (risperidone) and typical (haloperidol) antipsychotic agents on astroglial functions. Eur Arch Psychiatry Clin Neurosci 260:475–481
Rushlow WJ, Seah C, Sutton LP, Bjelica A, Rajakumar N (2009) Antipsychotics affect multiple calcium calmodulin dependent proteins. Neuroscience 161:877–886
Sagara Y (1998) Induction of reactive oxygen species in neurons by haloperidol. J Neurochem 71:1002–1012
Tarabová B, Nováková M, Lacinová L (2009) Haloperidol moderately inhibits cardiovascular L-type calcium current. Gen Physiol Biophys 28:249–259
Thompson AK, Mostafapour SP, Denlinger LC, Bleasdale JE, Fisher SK (1991) The aminosteroid U-73122 inhibits muscarinic receptor sequestration and phosphoinositide hydrolysis in SK-N-SH neuroblastoma cells. A role for Gp in receptor compartmentation. J Biol Chem 266:23856–23862
Tsien RY (1980) New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry 19:2396–2404
Van Esch GJ (1986) Toxicology of tert-butylhydroquinone (TBHQ). Food Chem Toxicol 24:1063–1065
Vinadé L, Gonçalves CA, Wofchuk S, Gottfried C, Rodnight R (1997) Evidence for a role for calcium ions in the dephosphorylation of glial fibrillary acidic protein (GFAP) in immature hippocampal slices and in astrocyte cultures from the rat. Brain Res Dev Brain Res 104:11–17
Wang CM, Chang YY, Sun SH (2003) Activation of P2X7 purinoceptor-stimulated TGF-beta 1 mRNA expression involves PKC/MAPK signalling pathway in a rat brain-derived type-2 astrocyte cell line, RBA-2. Cell Signal 15:1129–1137
Wei Z, Mousseau DD, Dai Y, Cao X, Li XM (2006) Haloperidol induces apoptosis via the sigma2 receptor system and Bcl-XS. Pharmacogenomics J 6:279–288
Zhang L, Yin JC, Yeh H, Ma NX, Lee G, Chen XA, Wang Y, Lin L, Chen L, Jin P, Wu GY, Chen G (2015) Small molecules efficiently reprogram human astroglial cells into functional neurons. Cell Stem Cell 17:735–747
Acknowledgments
This work was supported by the Department of Pharmacy and Master Program, College of Pharmacy and Health Care, Tajen University, Pingtung County 90741, Taiwan.
Author information
Authors and Affiliations
Contributions
SSH and WZL conceived and designed research. WZL conducted experiments. WZL contributed new reagents or analytical tools. SSH and WZL analyzed data. WZL wrote the manuscript. All authors read and approved the manuscript and all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Material
We have added supplemental original source data and “the effect of haloperidol on ROS production in GHAs and DI TNC1 cells” in “Supplementary Material” (DOC 44 kb)
Rights and permissions
About this article
Cite this article
Hsu, SS., Liang, WZ. Ca2+ signaling as a mechanism of haloperidol-induced cytotoxicity in human astrocytes and assessing the protective role of a Ca2+ chelator. Naunyn-Schmiedeberg's Arch Pharmacol 393, 2117–2127 (2020). https://doi.org/10.1007/s00210-020-01929-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01929-8