Abstract
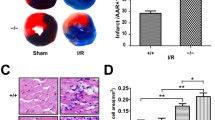
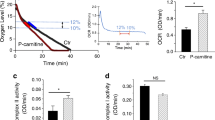
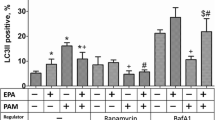
Depressed oxidation of long chain fatty acids (LCFA) in heart ischemia leads to acute accumulation of LCFA metabolites that impair the functioning of the mitochondria. We hypothesized that reduced activity of carnitine palmitoyltransferase-I (CPT-I) might activate peroxisomal LCFA oxidation and protect mitochondrial function in ischemia and reperfusion. In the present study, despite the long-term threefold reduction in L-carnitine content by 3-(2,2,2-trimethylhydrazinium)-propionate, the uptake and oxidation rates of LCFA in the heart in normoxia were not significantly influenced. The significant increase in PPARα and PGC1α nuclear content, observed in this study, were followed by increased expression of genes involved in peroxisomal fatty acid oxidation (FAO) which compensated for the limited CPT-I-dependent FA transport into the mitochondria. In ischemia followed by reperfusion, the redirection of LCFA oxidation from mitochondria to peroxisomes protected the mitochondria from the accumulation of LCFA. In turn, the recovery of FAO resulted in significant reduction of myocardial infarct size. In conclusion, the decreased L-carnitine content in the heart preserves its peroxisomal and mitochondrial function after ischemia and improves cardiac recovery during reperfusion. The functional interplay between the decrease in L-carnitine and the PPARα/PGC1α pathway-induced redirection of FA metabolism protects the mitochondria against LCFA overload and provides a foundation for novel cardioprotective mechanisms.







Similar content being viewed by others
References
Asaka N, Muranaka Y, Kirimoto T, Miyake H (1998) Cardioprotective profile of MET-88, an inhibitor of carnitine synthesis, and insulin during hypoxia in isolated perfused rat hearts. Fundam Clin Pharmacol 12:158–163
Beauloye C, Bertrand L, Horman S, Hue L (2011) AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc Res 90:224–233
Dambrova M, Liepinsh E, Kalvinsh I (2002) Mildronate: cardioprotective action through carnitine-lowering effect. Trends Cardiovasc Med 12:275–279
Dambrova M, Cirule H, Svalbe B, Zvejniece L, Pugovichs O, Zorenko T, Kalvinsh I, Liepinsh E, Belozertseva I (2008) Effect of inhibiting carnitine biosynthesis on male rat sexual performance. Physiol Behav 95:341–347
Degrace P, Demizieux L, Gresti J, Tsoko M, Andre A, Demaison L, Clouet P (2004) Fatty acid oxidation and related gene expression in heart depleted of carnitine by mildronate treatment in the rat. Mol Cell Biochem 258:171–182
Doenst T, Richwine RT, Bray MS, Goodwin GW, Frazier OH, Taegtmeyer H (1999) Insulin improves functional and metabolic recovery of reperfused working rat heart. Ann Thorac Surg 67:1682–1688
Duncan JG (2011) Peroxisome proliferator activated receptor-alpha (PPARalpha) and PPAR gamma coactivator-1alpha (PGC-1alpha) regulation of cardiac metabolism in diabetes. Pediatr Cardiol 32:323–328
Finck BN, Kelly DP (2007) Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation 115:2540–2548
Gupta S, Young D, Maitra RK, Gupta A, Popovic ZB, Yong SL, Mahajan A, Wang Q, Sen S (2008) Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB. J Mol Biol 375:637–649
Harrison EH, Walusimbi-Kisitu M (1988) Properties and subcellular localization of myocardial fatty acyl-coenzyme A oxidase. Am J Physiol 255:H441–H445
Hayashi Y, Tajima K, Kirimoto T, Miyake H, Matsuura N (2000) Cardioprotective effects of MET-88, a gamma-butyrobetaine hydroxylase inhibitor, on cardiac dysfunction induced by ischemia/reperfusion in isolated rat hearts. Pharmacology 61:238–243
Kim M, Tian R (2011) Targeting AMPK for cardiac protection: opportunities and challenges. J Mol Cell Cardiol 51:548–553
Kono K, Kamijo Y, Hora K, Takahashi K, Higuchi M, Kiyosawa K, Shigematsu H, Gonzalez FJ, Aoyama T (2009) PPAR{alpha} attenuates the proinflammatory response in activated mesangial cells. Am J Physiol Renal Physiol 296:F328–F336
Korge P, Honda HM, Weiss JN (2003) Effects of fatty acids in isolated mitochondria: implications for ischemic injury and cardioprotection. Am J Physiol Heart Circ Physiol 285:H259–H269
Kuka J, Vilskersts R, Cirule H, Makrecka M, Pugovics O, Kalvinsh I, Dambrova M, Liepinsh E (2012) The cardioprotective effect of mildronate is diminished after co-treatment with L-carnitine. J Cardiovasc Pharmacol Ther 17:215–222
Liepinsh E, Vilskersts R, Loca D, Kirjanova O, Pugovichs O, Kalvinsh I, Dambrova M (2006) Mildronate, an inhibitor of carnitine biosynthesis, induces an increase in gamma-butyrobetaine contents and cardioprotection in isolated rat heart infarction. J Cardiovasc Pharmacol 48:314–319
Liepinsh E, Vilskersts R, Zvejniece L, Svalbe B, Skapare E, Kuka J, Cirule H, Grinberga S, Kalvinsh I, Dambrova M (2009) Protective effects of mildronate in an experimental model of type 2 diabetes in Goto-Kakizaki rats. Br J Pharmacol 157:1549–1556
Liepinsh E, Kalvinsh I, Dambrova M (2011a) The regulation of energy metabolism pathways through L-carnitine homeostasis. In: Croniger C (ed) Role of the adipocyte in development of type 2 diabetes. InTech, Rijeka, pp 107–128
Liepinsh E, Skapare E, Svalbe B, Makrecka M, Cirule H, Dambrova M (2011b) Anti-diabetic effects of mildronate alone or in combination with metformin in obese Zucker rats. Eur J Pharmacol 658:277–283
Liepinsh E, Skapare E, Svalbe B, Makrecka M, Cirule H, Dambrova M (2011c) Anti-diabetic effects of mildronate alone or in combination with metformin in obese Zucker rats. Eur J Pharmacol 658:277–283
Lopaschuk GD, Barr RL (1997) Measurements of fatty acid and carbohydrate metabolism in the isolated working rat heart. Mol Cell Biochem 172:137–147
Luiken JJ, Niessen HE, Coort SL, Hoebers N, Coumans WA, Schwenk RW, Bonen A, Glatz JF (2009) Etomoxir-induced partial carnitine palmitoyltransferase-I (CPT-I) inhibition in vivo does not alter cardiac long-chain fatty acid uptake and oxidation rates. Biochem J 419:447–455
Poirier Y, Antonenkov VD, Glumoff T, Hiltunen JK (2006) Peroxisomal beta-oxidation—a metabolic pathway with multiple functions. Biochim Biophys Acta 1763:1413–1426
Ravingerova T, Adameova A, Carnicka S, Nemcekova M, Kelly T, Matejikova J, Galatou E, Barlaka E, Lazou A (2011) The role of PPAR in myocardial response to ischemia in normal and diseased heart. Gen Physiol Biophys 30:329–341
Schurch R, Todesco L, Novakova K, Mevissen M, Stieger B, Krahenbuhl S (2010) The plasma carnitine concentration regulates renal OCTN2 expression and carnitine transport in rats. Eur J Pharmacol 635:171–176
Sebastian D, Guitart M, Garcia-Martinez C, Mauvezin C, Orellana-Gavalda JM, Serra D, Gomez-Foix AM, Hegardt FG, Asins G (2009) Novel role of FATP1 in mitochondrial fatty acid oxidation in skeletal muscle cells. J Lipid Res 50:1789–1799
Shriver LP, Manchester M (2011) Inhibition of fatty acid metabolism ameliorates disease activity in an animal model of multiple sclerosis. Sci Rep 1:79
Sparagna GC, Hickson-Bick DL, Buja LM, McMillin JB (2000) A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol 279:H2124–H2132
Srere PA (1963) Citryl-CoA an substrate for the citrate-cleavage enzyme. Biochim Biophys Acta 73:523–525
Sugden MC, Zariwala MG, Holness MJ (2009) PPARs and the orchestration of metabolic fuel selection. Pharmacol Res 60:141–150
Tominaga H, Katoh H, Odagiri K, Takeuchi Y, Kawashima H, Saotome M, Urushida T, Satoh H, Hayashi H (2008) Different effects of palmitoyl-L-carnitine and palmitoyl-CoA on mitochondrial function in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 295:H105–H112
Ussher JR, Wang W, Gandhi M, Keung W, Samokhvalov V, Oka T, Wagg CS, Jaswal JS, Harris RA, Clanachan AS, Dyck JR, Lopaschuk GD (2012) Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc Res 94:359–369
Vilskersts R, Liepinsh E, Mateuszuk L, Grinberga S, Kalvinsh I, Chlopicki S, Dambrova M (2009) Mildronate, a regulator of energy metabolism, reduces atherosclerosis in apoE/LDLR−/− mice. Pharmacology 83:287–293
Wang W, Lopaschuk GD (2007) Metabolic therapy for the treatment of ischemic heart disease: reality and expectations. Expert Rev Cardiovasc Ther 5:1123–1134
Wheeler CR, Salzman JA, Elsayed NM, Omaye ST, Korte DW Jr (1990) Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal Biochem 184:193–199
Yue TL, Bao W, Jucker BM, Gu JL, Romanic AM, Brown PJ, Cui J, Thudium DT, Boyce R, Burns-Kurtis CL, Mirabile RC, Aravindhan K, Ohlstein EH (2003) Activation of peroxisome proliferator-activated receptor-alpha protects the heart from ischemia/reperfusion injury. Circulation 108:2393–2399
Acknowledgments
This research was supported by the Taiho Foundation, ERDF grant no. 2010/0234/2DP/2.1.1.1.0/10/APIA/VIAA/063, L’Oréal Latvian fellowship “For Women in Science”, and the European Social Fund agreement no. 009/0147/1DP/1.1.2.1.2/09/IPIA/VIAA/009.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1105 kb)
Rights and permissions
About this article
Cite this article
Liepinsh, E., Skapare, E., Kuka, J. et al. Activated peroxisomal fatty acid metabolism improves cardiac recovery in ischemia-reperfusion. Naunyn-Schmiedeberg's Arch Pharmacol 386, 541–550 (2013). https://doi.org/10.1007/s00210-013-0849-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-013-0849-0