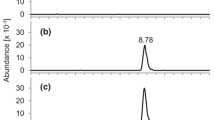
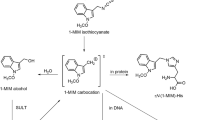
Abstract
Novel aminonaphthylcysteine (ANC) adducts, formed via naphthylnitrenium ions and/or their metabolic precursors in the biotransformation of naphthylamines (NA) and nitronaphthalenes (NN), were identified and quantified in globin of rats dosed intraperitoneally with 0.16 mmol/kg b.w. of 1-NA, 1-NN, 2-NA and 2-NN. Using HPLC-ESI-MS2 analysis of the globin hydrolysates, S-(1-amino-2-naphthyl)cysteine (1A2NC) together with S-(4-amino-1-naphthyl)cysteine (4A1NC) were found in rats given 1-NA or 1-NN, and S-(2-amino-1-naphthyl)cysteine (2A1NC) in those given 2-NA or 2-NN. The highest level of ANC was produced by the most mutagenic and carcinogenic isomer 2-NA (35.8 ± 5.4 nmol/g globin). The ratio of ANC adduct levels for 1-NA, 1-NN, 2-NA and 2-NN was 1:2:100:3, respectively. Notably, the ratio of 1A2NC:4A1NC in globin of rats dosed with 1-NA and 1-NN differed significantly (2:98 versus 16:84 respectively), indicating differences in mechanism of the adduct formation. Moreover, aminonaphthylmercapturic acids, formed via conjugation of naphthylnitrenium ions and/or their metabolic precursors with glutathione, were identified in the rat urine. Their amounts excreted after dosing rats with 1-NA, 1-NN, 2-NA and 2-NN were in the ratio 1:100:40:2, respectively. For all four compounds tested, haemoglobin binding index for ANC was several-fold higher than that for the sulphinamide adducts, generated via nitrosoarene metabolites. Due to involvement of electrophilic intermediates in their formation, ANC adducts in globin may become toxicologically more relevant biomarkers of cumulative exposure to carcinogenic or non-carcinogenic arylamines and nitroarenes than the currently used sulphinamide adducts.






Similar content being viewed by others
References
Boyland E, Manson D, Nery R (1963) Mercapturic acids as metabolites of aniline and 2-naphthylamine. Biochem J 86:263–271
Chiang TA, Pei-Fen W, Ying LS, Wang LF, Ko YC (1999) Mutagenicity and aromatic amine content of fumes from heated cooking oils produced in Taiwan. Food Chem Toxicol 37:125–134. https://doi.org/10.1016/s0278-6915(98)00081-7
Chou HC, Lang NC, Kadlubar FF (1995) Metabolic activation of N-hydroxy arylamines and N-hydroxy heterocyclic amines by human sulfotransferase(s). Cancer Res 55:525–529
Debnath AK, Lopez de Compadre RL, Debnath G, Shusterman AJ, Hansch C (1991) Structure-activity relationship of mutagenic aromatic and heteroaromatic nitro compounds. Correlation with molecular orbital energies and hydrophobicity. J Med Chem 34:786–797. https://doi.org/10.1021/jm00106a046
El-Bayoumi K, Lavoie EJ, Hecht SS, Fow EA, Hoffmann D (1981) The influence of methyl substitution to the mutagenicity of nitronaphthalenes and nitrobiphenyls. Mutat Res 81:143–153. https://doi.org/10.1016/0027-5107(81)90029-4
Feltes J, Levsen K, Volmer D, Spiekermann M (1990) Gas chromatographic and mass spectrometric determination of nitroaromatics in water. J Chromatogr 518:21–40
Ford GP, Herman PS (1992) Relative stabilities of nitrenium ions derived from polycyclic aromatic amines. Relationship to mutagenicity. Chem Biol Interact 81(1–2):1–18. https://doi.org/10.1016/0009-2797(92)90023-e
Ford GP, Thompson JW (1999) Regiochemistry of nucleophilic attack by the guanine 2-amino group at the ring positions of nitrenium ions derived from carcinogenic polycyclic arylamines and nitroarenes: molecular orbital calculations and simple models. Chem Res Toxicol 12:53–59. https://doi.org/10.1021/tx9801460
Freeman HS (2013) Aromatic amines: use in azo dye chemistry. Front Biosci 18:145–164. https://doi.org/10.2741/4093
Fu PP (1990) Metabolism of nitro-polycyclic aromatic compounds. Drug Metab Rev 22:209–268. https://doi.org/10.3109/03602539009041085
Gresh N (1982) Carcinogenicity of naphthylamines: A theoretical study of the stacking interaction of 1- and 2-naphthylnitrenium ions with guanine, adenine, and the dinucleoside monophosphate cytidyl-(3'-5')-guanosine. Int J Quant Chem 22:37–48
Hix C, Oglesby L, Macnair P, Sieg M, Langenbach R (1983) Bovine bladder and liver cell and homogenate-mediated mutagenesis of Salmonella typhimurium with aromatic amines. Carcinogenesis 4(11):1401–1407. https://doi.org/10.1093/carcin/4.11.1401
IARC (2012) IARC monographs on the evaluation of carcinogenic risks to humans. Chemical agents and related occupations. Vol. 100F A review of human carcinogens. IARC, Lyon
Khyatiben VP, Ting-Lan C, Amin EA, Turesky RJ (2016) Methemoglobin formation and characterization of hemoglobin adducts of carcinogenic aromatic amines and heterocyclic aromatic amines. Chem Res Toxicol 29:255–269. https://doi.org/10.1021/acs.chemrestox.5b00418
Linhart I, Mráz J, Hanzlíková I, Šilhánková A, Frantík E, Himl M (2012) Carcinogenic 3-nitrobenzanthrone but not 2-nitrobenzanthrone is metabolised to an unusual mercapturic acid in rats. Toxicol Lett 208:246–253. https://doi.org/10.1016/j.toxlet.2011.11.017
Linhart I, Hanzlíková I, Mráz J, Dušková Š (2017) S-(3-Aminobenzanthron-2-yl)cysteine in the globin of rats as a novel type of adduct and possible biomarker of exposure to 3-nitrobenzanthrone, a potent environmental carcinogen. Arch Toxicol 91:3317–3325. https://doi.org/10.1007/s00204-017-1943-8
Mermelstein R, McCoy EC, Rosenkraz HS (1985) The mutagenic properties of nitroarenes: structure activity relationships. In: Rickert DE (ed) Toxicity of nitroaromatic compounds. Hemispehere Publishing Co, Washington, pp 207–208
Mráz J, Hanzlíková I, Moulisová A, Dušková Š, Hejl K, Bednářová A, Linhart I (2016) Hydrolytic cleavage products of globin adducts in urine as possible biomarkers of cumulative dose. Proof of concept using styrene oxide as a model adduct-forming compound. Chem Res Toxicol 29(4):676–686. https://doi.org/10.1021/acs.chemrestox.5b00518
Neumann H-G, Birner G, Kowallik P, Schütze D, Zwirner-Baier I (1993) Hemoglobin adducts of N-substituted aryl compounds in exposure control and risk assessment. Environ Health Perspect 99:65–69. https://doi.org/10.1289/ehp.939965
Novak M, Chakraborty M (2011) N-Arylhydroxylamines and chemical carcinogenicity. In: Rappoport Z, Liebman JF (eds) The chemistry of hydroxylamines, oximes and hydroxamic acids, vol 2. Wiley, New York, pp 577–621
Novak M, Rajagopal S (2002) Correlations of nitrenium ion selectivities with quantitative mutagenicity and carcinogenicity of the corresponding amines. Chem Res Toxicol 15:1495–1503. https://doi.org/10.1021/tx025584s
Purchase IF, Kalinowski AE, Ishmael J, Wilson J, Gore CW, Chart IS (1981) Lifetime carcinogenicity study of 1- and 2-naphthylamine in dogs. Br J Cancer 44(6):892–901. https://doi.org/10.1038/bjc.1981.289
Radomski JL, Deichmann W, Altman LH, Radomski T (1980) Failure of pure 1-naphthylamine to induce bladder tumors in dogs. Cancer Res 40:3537–3539
Rosenkranz HS, Mermelstein R (1985) Mutagenicity and genotoxicity of nitroarenes. All nitro-containing chemicals were not created equal. Mutat Res 114:217–267
Sabbioni G (1994) Hemoglobin binding of arylamines and nitroarenes: molecular dosimetry and quantitative structure-activity relationships. Environ Health Perspect 102(Suppl. 6):61–67. https://doi.org/10.1289/ehp.94102s661
Sabbioni G (2017) Hemoglobin adducts and urinary metabolites of arylamines and nitroarenes. Chem Res Toxicol 30:1733–1766. https://doi.org/10.1021/acs.chemrestox.7b00111
Shamovsky I, Ripa L, Blomberg N, Eriksson LA, Hansen P, Mee C, Tyrchan C, O'Donovan M, Sjö P (2012) Theoretical studies of chemical reactivity of metabolically activated forms of aromatic amines toward DNA. Chem Res Toxicol 25:2236–2252. https://doi.org/10.1021/tx300313b
Suzuki J, Meguro S-I, Morita O, Hirayama S, Suzuki S (1989) Comparison of in vivo binding of aromatic nitro and amino compounds to rat hemoglobin. Biochem Pharmacol 38:3511–3519. https://doi.org/10.1016/0006-2952(89)90122-6
Tatsumi K, Kitamura S, Narai N (1986) Reductive metabolism of aromatic nitro compounds including carcinogens by rabbit liver preparations. Cancer Res 46:1089–1093
Turesky RJ (2010) Aromatic amines and heterocyclic aromatic amines: from tobacco smoke to food mutagens. In: Geacintov NE, Broyde S (eds) The chemical biology of DNA damage. Wiley, Weinheim, pp 157–182
Acknowledgements
The study was funded by the institutional support DRO (National Institute of Public Health—NIPH, IN 75010330) from the Ministry of Health of the Czech Republic and Program of long-term development of the University of Chemistry and Technology, Prague. Purchase of the principal analytical instrument, Orbitrap Q Exactive mass spectrometer, was supported by European Regional Development Fund (ERDF), Integrated Operational Programme Reg. No. CZ.1.06/3.2.01/11.08435. Skilled technical assistance of Radka Vajtrová is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Linhart, I., Hanzlíková, I., Mráz, J. et al. Novel aminoarylcysteine adducts in globin of rats dosed with naphthylamine and nitronaphthalene isomers. Arch Toxicol 95, 79–89 (2021). https://doi.org/10.1007/s00204-020-02907-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02907-y