Abstract
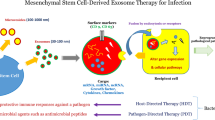
Fungal infections concomitant with biofilms can demonstrate an elevated capacity to withstand substantially higher concentrations of antifungal agents, contrasted with infectious diseases caused by planktonic cells. This inherent resilience intrinsic to biofilm-associated infections engenders a formidable impediment to effective therapeutic interventions. The different mechanisms that are associated with the intrinsic resistance of Candida species encompass drug sequestration by the matrix, drug efflux pumps, stress response cell density, and the presence of persister cells. These persisters, a subset of fungi capable of surviving hostile conditions, pose a remarkable challenge in clinical settings in virtue of their resistance to conventional antifungal therapies. Hence, an exigent imperative has arisen for the development of novel antifungal therapeutics with specific targeting capabilities focused on these pathogenic persisters. On a global scale, fungal persistence and their resistance within biofilms generate an urgent clinical need for investigating recently introduced therapeutic strategies. This review delves into the unique characteristics of Mesenchymal stem/stromal cells (MSCs) and their secreted exosomes, which notably exhibit immunomodulatory and regenerative properties. By comprehensively assessing the current literature and ongoing research in this field, this review sheds light on the plausible mechanisms by which MSCs and their exosomes can be harnessed to selectively target fungal persisters. Additionally, prospective approaches in the use of cell-based therapeutic modalities are examined, emphasizing the importance of further research to overcome the enigmatic fungal persistence.



Similar content being viewed by others
Data availability
Not applicable.
References
AlMaghrabi RS, Al-Musawi T, Albaksami O, Subhi AL, Fakih RE, Stone NR (2023) Challenges in the management of invasive fungal infections in the Middle East: expert opinion to optimize management using a multidisciplinary approach. Cureus 15:e44356
Arendrup MC (2010) Epidemiology of invasive candidiasis. Curr Opin Crit Care 16:445–452
Ashrit P, Sadanandan B, Shetty K, Vaniyamparambath V (2022) Polymicrobial biofilm dynamics of multidrug-resistant Candida albicans and Ampicillin-resistant Escherichia coli and antimicrobial inhibition by aqueous garlic extract. Antibiotics (Basel) 11:34
Baharlooi H, Azimi M, Salehi Z, Izad M (2020) Mesenchymal stem cell-derived exosomes: a promising therapeutic ace card to address autoimmune diseases. Int J Stem Cells 13:13–23
Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, Yu B, Chen X, Li X, Zhang X (2017) Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep 7:4323
Baillie GS, Douglas LJ (2000) Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Chemother 46:397–403
Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S (2004) Bacterial persistence as a phenotypic switch. Science 305:1622–1625
Barantsevich N, Barantsevich E (2022) Diagnosis and treatment of invasive Candidiasis. Antibiotics 11:718
Benedict K, Whitham HK, Jackson BR (2022) Economic Burden of Fungal Diseases in the United States. Open Forum Infect Dis 9:4
Bink A, Vandenbosch D, Coenye T, Nelis H, Cammue BP, Thevissen K (2011) Superoxide dismutases are involved in Candida albicans biofilm persistence against miconazole. Antimicrob Agents Chemother 55:4033–4037
Bongomin F, Gago S, Oladele RO, Denning DW (2017) Global and multi-national prevalence of fungal diseases—Estimate Precision. J Fungi 3:57
Bonhomme J, d’Enfert C (2013) Candida albicans biofilms: building a heterogeneous, drug-tolerant environment. Curr Opin Microbiol 16:398–403
Brauner A, Fridman O, Gefen O, Balaban NQ (2016) Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 14:320–330
Brauner A, Alvendal C, Chromek M, Stopsack KH, Ehrström S, Schröder JM, Bohm-Starke N (2018) Psoriasin, a novel anti-Candida albicans adhesin. J Mol Med 96:537–545
Brown AJP (2023) Fungal resilience and host–pathogen interactions: future perspectives and opportunities. Parasite Immunol 45:e12946
Camilli G, Tabouret G, Quintin J (2018) The complexity of fungal β-glucan in health and disease: effects on the mononuclear phagocyte system. Front Immunol 9:673
Cavalheiro M, Teixeira MC (2018) Candida biofilms: threats, challenges, and promising strategies. Front Med (lausanne) 5:28
Chandra J, Mukherjee PK (2015) Candida biofilms: development, architecture, and resistance. Microbiol Spectr 3:34
Chen K, Gong W, Huang J, Yoshimura T, Wang JM (2021) The potentials of short fragments of human anti-microbial peptide LL-37 as a novel therapeutic modality for diseases. FBL 26:1362–1372
Chen SC, Slavin MA, Sorrell TC (2011) Echinocandin antifungal drugs in fungal infections: a comparison. Drugs 71:11–41
Cho BS, Kim JO, Ha DH, Yi YW (2018) Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res Ther 9:187
Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ (2011) Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood 118:330–338
Chow L, Johnson V, Impastato R, Coy J, Strumpf A, Dow S (2020) Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells. Stem Cells Transl Med 9:235–249
Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C, Noël D (2018) Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 8:1399–1410
Cruz FF, Borg ZD, Goodwin M, Sokocevic D, Wagner DE, Coffey A, Antunes M, Robinson KL, Mitsialis SA, Kourembanas S, Thane K, Hoffman AM, McKenna DH, Rocco PR, Weiss DJ (2015) Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl Med 4:1302–1316
de Godoy MA, Saraiva LM, de Carvalho LRP, Vasconcelos-Dos-Santos A, Beiral HJV, Ramos AB, Silva LRP, Leal RB, Monteiro VHS, Braga CV, de Araujo-Silva CA, Sinis LC, Bodart-Santos V, Kasai-Brunswick TH, Alcantara CL, Lima A, da Cunha ESNL, Galina A, Vieyra A, De Felice FG, Mendez-Otero R, Ferreira ST (2018) Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. J Biol Chem 293:1957–1975
de Oca EPM (2013) Antimicrobial peptide elicitors: New hope for the post-antibiotic era. Innate Immun 19:227–241
DelaRosa O, Lombardo E (2010) Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflamm 2010:865601
Delarze E, Sanglard D (2015) Defining the frontiers between antifungal resistance, tolerance and the concept of persistence. Drug Resist Updates 23:12–19
Dominguez E, Zarnowski R, Sanchez H, Covelli AS, Westler WM, Azadi P, Nett J, Mitchell AP, Andes DR (2018) Conservation and divergence in the Candida species biofilm matrix mannan-glucan complex structure, function, and genetic control. Bio 9:e00451
Fanning S, Mitchell AP (2012) Fungal biofilms. PLoS Pathog 8:e1002585
Fernandes T, Silva S, Henriques M (2016) Effect of Voriconazole on Candida tropicalis Biofilms: Relation with ERG Genes Expression. Mycopathologia 181:643–651
Fisher MC, Alastruey-Izquierdo A, Berman J, Bicanic T, Bignell EM, Bowyer P, Bromley M, Brüggemann R, Garber G, Cornely OA, Gurr SJ, Harrison TS, Kuijper E, Rhodes J, Sheppard DC, Warris A, White PL, Xu J, Zwaan B, Verweij PE (2022) Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol 20:557–571
Fisher RA, Gollan B, Helaine S (2017) Persistent bacterial infections and persister cells. Nat Rev Microbiol 15:453–464
Fusco A, Savio V, Donniacuo M, Perfetto B, Donnarumma G (2021) Antimicrobial peptides human beta-defensin-2 and -3 protect the gut during candida albicans infections enhancing the intestinal barrier integrity: in vitro study. Front Cell Infect Microbiol 11:666900
Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM (2003) Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med 197:1107–1117
Garcia-Cuesta C, Sarrion-Pérez MG, Bagán JV (2014) Current treatment of oral candidiasis: A literature review. J Clin Exp Dent 6:e576-582
Geißel B, Loiko V, Klugherz I, Zhu Z, Wagener N, Kurzai O, van den Hondel C, Wagener J (2018) Azole-induced cell wall carbohydrate patches kill Aspergillus fumigatus. Nat Commun 9:3098
Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV (2020) Mesenchymal stem cell-derived extracellular vesicles: challenges in clinical applications. Front Cell Dev Biol 8:149
Gulati M, Nobile CJ (2016) Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect 18:310–321
Gutiérrez-Correa M, Ludeña Y, Ramage G, Villena GK (2012) Recent advances on filamentous fungal biofilms for industrial uses. Appl Biochem Biotechnol 167:1235–1253
Halder LD, Jo EAH, Hasan MZ, Ferreira-Gomes M, Krüger T, Westermann M, Palme DI, Rambach G, Beyersdorf N, Speth C, Jacobsen ID, Kniemeyer O, Jungnickel B, Zipfel PF, Skerka C (2020) Immune modulation by complement receptor 3-dependent human monocyte TGF-β1-transporting vesicles. Nat Commun 11:2331
Harder J, Schröder J-M (2005) Psoriatic scales: a promising source for the isolation of human skin-derived antimicrobial proteins. J Leukoc Biol 77:476–486
Hawser SP, Douglas LJ (1994) Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect Immun 62:915–921
Hawser SP, Baillie GS, Douglas LJ (1998) Production of extracellular matrix by Candida albicans biofilms. J Med Microbiol 47:253–256
Herre J, Marshall AS, Caron E, Edwards AD, Williams DL, Schweighoffer E, Tybulewicz V (2004) Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 104:4038–4045
Houšť J, Spížek J, Havlíček V (2020) Antifungal Drugs. Metabolites 10:106
Ikonomova SP, Moghaddam-Taaheri P, Wang Y, Doolin MT, Stroka KM, Hube B, Karlsson AJ (2020) Effects of histatin 5 modifications on antifungal activity and kinetics of proteolysis. Protein Sci 29:480–493
Jabra-Rizk MA, Falkler WA, Meiller TF (2004) Fungal biofilms and drug resistance. Emerg Infect Dis 10:14–19
Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC, Fan Y, Wang Y, Wang NS (2016) Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther 7:24
Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M, Ochiya T (2013) Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep 3:1197
Keshtkar S, Kaviani M, Soleimanian S, Azarpira N, Asvar Z, Pakbaz S (2022) Stem cell-derived exosome as potential therapeutics for microbial diseases. Front Microbiol 12:23
Khurshid Z, Najeeb S, Mali M, Moin SF, Raza SQ, Zohaib S, Sefat F, Zafar MS (2017) Histatin peptides: pharmacological functions and their applications in dentistry. Saudi Pharm J 25:25–31
Konopka K, Dorocka-Bobkowska B, Gebremedhin S, Düzgüneş N (2010) Susceptibility of Candida biofilms to histatin 5 and fluconazole. Antonie Van Leeuwenhoek 97:413–417
Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B (2014) MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28:970–973
Kotani H, Koshizuka T, Matsubara K, Nishiyama K, Sugiyama T, Suzutani T (2020) Relationship between human β-defensin 2 and the vaginal environment. Jpn J Infect Dis 73:214–220
Kumar A, Zarychanski R, Pisipati A, Kumar A, Kethireddy S, Bow EJ (2018) Fungicidal versus fungistatic therapy of invasive Candida infection in non-neutropenic adults: a meta-analysis. Mycology 9:116–128
Lamfon H, Porter SR, McCullough M, Pratten J (2004) Susceptibility of Candida albicans biofilms grown in a constant depth film fermentor to chlorhexidine, fluconazole and miconazole: a longitudinal study. J Antimicrob Chemother 53:383–385
Laso-García F, Ramos-Cejudo J, Carrillo-Salinas FJ, Otero-Ortega L, Feliú A, Gómez-de Frutos M, Mecha M, Díez-Tejedor E, Guaza C, Gutiérrez-Fernández M (2018) Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS ONE 13:e0202590
Lee Y, Puumala E, Robbins N, Cowen LE (2021) Antifungal drug resistance: molecular mechanisms in Candida albicans and beyond. Chem Rev 121:3390–3411
Lewis K (2008) Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol 322:107–131
Lewis K (2010) Persister cells. Annu Rev Microbiol 64:357–372
Li K-L, Li J-Y, Xie G-L, Ma X-Y (2021) Exosomes released from human bone marrow–derived mesenchymal stem cell attenuate acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation in mice. Front Cell Develop Biol 9:589
Li P, Seneviratne CJ, Alpi E, Vizcaino JA, Jin L (2015a) Delicate metabolic control and coordinated stress response critically determine antifungal tolerance of Candida albicans biofilm persisters. Antimicrob Agents Chemother 59:6101–6112
Li R, Zhang L, Zhang H, Yi Y, Wang L, Chen L, Zhang L (2017) Protective effect of a novel antifungal peptide derived from human chromogranin a on the immunity of mice infected with Candida krusei. Exp Ther Med 13:2429–2434
Li RF, Lu YL, Lu YB, Zhang HR, Huang L, Yin Y, Zhang L, Liu S, Lu Z, Sun Y (2015b) Antiproliferative effect and characterization of a novel antifungal peptide derived from human Chromogranin A. Exp Ther Med 10:2289–2294
Li Z, Liu F, He X, Yang X, Shan F, Feng J (2019) Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharmacol 67:268–280
Liotta F, Angeli R, Cosmi L, Filì L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F (2008) Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells 26:279–289
Lopes JP, Lionakis MS (2022) Pathogenesis and virulence of Candida albicans. Virulence 13:89–121
Ma ZJ, Yang JJ, Lu YB, Liu ZY, Wang XX (2020) Mesenchymal stem cell-derived exosomes: toward cell-free therapeutic strategies in regenerative medicine. World J Stem Cells 12:814–840
Mardpour S, Ghanian MH, Sadeghi-Abandansari H, Mardpour S, Nazari A, Shekari F, Baharvand H (2019) Hydrogel-mediated sustained systemic delivery of mesenchymal stem cell-derived extracellular vesicles improves hepatic regeneration in chronic liver failure. ACS Appl Mater Interfaces 11:37421–37433
Marx C, Gardner S, Harman RM, Van de Walle GR (2020) The mesenchymal stromal cell secretome impairs methicillin-resistant Staphylococcus aureus biofilms via cysteine protease activity in the equine model. Stem Cells Transl Med 9:746–757
Mathé L, Van Dijck P (2013) Recent insights into Candida albicans biofilm resistance mechanisms. Curr Genet 59:251–264
Matsuzaki G, Umemura M (2007) Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol 51:1139–1147
McBride JD, Rodriguez-Menocal L, Candanedo A, Guzman W, Garcia-Contreras M, Badiavas EV (2018) Dual mechanism of type VII collagen transfer by bone marrow mesenchymal stem cell extracellular vesicles to recessive dystrophic epidermolysis bullosa fibroblasts. Biochimie 155:50–58
McCarthy MW, Walsh TJ (2017) Drugs currently under investigation for the treatment of invasive candidiasis. Expert Opin Investig Drugs 26:825–831
Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, Marszalek JR, Maitra A, Yee C, Rezvani K, Shpall E, LeBleu VS, Kalluri R (2018) Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 3:99623
Mitchell KF, Zarnowski R, Sanchez H, Edward JA, Reinicke EL, Nett JE, Mitchell AP, Andes DR (2015) Community participation in biofilm matrix assembly and function. Proc Natl Acad Sci 112:4092–4097
Moghaddam-Taaheri P, Leissa JA, Eppler HB, Jewell CM, Karlsson AJ (2021) Histatin 5 variant reduces Candida albicans biofilm viability and inhibits biofilm formation. Fungal Genet Biol 149:103529
Muzny CA, Schwebke JR (2015) Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis 61:601–606
Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad AN, Essa W, Adel H (2016) Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res 20:21
Nauta AJ, Fibbe WE (2007) Immunomodulatory properties of mesenchymal stromal cells. Blood 110:3499–3506
Nijnik A, Hancock RE (2009) The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr Opin Hematol 16:41–47
Nobile CJ, Johnson AD (2015) Candida albicans biofilms and human disease. Annu Rev Microbiol 69:71–92
Norooznezhad AH, Yarani R, Payandeh M, Hoseinkhani Z, Kiani S, Taghizadeh E, Thakor AS, Mansouri K (2022) Human placental mesenchymal stromal cell-derived exosome-enriched extracellular vesicles for chronic cutaneous graft-versus-host disease: A case report. J Cell Mol Med 26:588–592
Opitz CA, Litzenburger UM, Lutz C, Lanz TV, Tritschler I, Köppel A, Tolosa E, Hoberg M, Anderl J, Aicher WK, Weller M, Wick W, Platten M (2009) Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells 27:909–919
Parums DV (2022) Editorial: The World Health Organization (WHO) fungal priority pathogens list in response to emerging fungal pathogens during the COVID-19 pandemic. Med Sci Monit 28:e939088
Patil A, Majumdar S (2017) Echinocandins in antifungal pharmacotherapy. J Pharm Pharmacol 69:1635–1660
Perez-Rodriguez A, Eraso E, Quindós G, Mateo E (2022) Antimicrobial Peptides with Anti-Candida Activity. Int J Mol Sci 23:9264
Pierce LM, Kurata WE (2021) Priming With Toll-Like Receptor 3 Agonist Poly(I:C) enhances content of innate immune defense proteins but not microRNAs in human mesenchymal stem cell-derived extracellular vesicles. Front Cell Dev Biol 9:676356
Prasad R, De Wergifosse P, Goffeau A, Balzi E (1995) Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet 27:320–329
Rather IA, Sabir JSM, Asseri AH, Ali S (2022) Antifungal Activity of Human Cathelicidin LL-37, a membrane disrupting peptide, by triggering oxidative stress and cell cycle arrest in Candida auris. J Fungi 8:204
Rex JH, Bennett JE, Sugar AM, Pappas PG, van der Horst CM, Edwards JE, Washburn RG, Scheld WM, Karchmer AW, Dine AP et al (1994) A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med 331:1325–1330
Rodrigues CF, Silva S, Azeredo J, Henriques M (2016) Candida glabrata’s recurrent infections: biofilm formation during Amphotericin B treatment. Lett Appl Microbiol 63:77–81
Rokas A (2022) Evolution of the human pathogenic lifestyle in fungi. Nat Microbiol 7:607–619
Rosenberg A, Ene IV, Bibi M, Zakin S, Segal ES, Ziv N, Dahan AM, Colombo AL, Bennett RJ, Berman J (2018) Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia. Nat Commun 9:2470
Saberpour M, Bakhshi B, Najar-Peerayeh S (2020) Evaluation of the antimicrobial and antibiofilm effect of chitosan nanoparticles as carrier for supernatant of mesenchymal stem cells on multidrug-resistant Vibrio cholerae. Infect Drug Resist 13:2251–2260
Scarsini M, Tomasinsig L, Arzese A, D’Este F, Oro D, Skerlavaj B (2015) Antifungal activity of cathelicidin peptides against planktonic and biofilm cultures of Candida species isolated from vaginal infections. Peptides 71:211–221
Schinocca C, Rizzo C, Fasano S, Grasso G, La Barbera L, Ciccia F, Guggino G (2021) Role of the IL-23/IL-17 pathway in rheumatic diseases: an overview. Front Immunol 12:637829
Schmidt S, Tramsen L, Schneider A, Schubert R, Balan A, Degistirici Ö, Meisel R, Lehrnbecher T (2017) Impact of human mesenchymal stromal cells on antifungal host response against Aspergillus fumigatus. Oncotarget 8:95495–95503
Schneider J, Mateo E, Marcos-Arias C, Eiró N, Vizoso F, Pérez-Fernández R, Eraso E, Quindós G (2018) Antifungal activity of the human uterine cervical stem cells conditioned medium (hUCESC-CM) against Candida albicans and other medically relevant species of Candida. Front Microbiol 9:2818
Scriven JE, Tenforde MW, Levitz SM, Jarvis JN (2017) Modulating host immune responses to fight invasive fungal infections. Curr Opin Microbiol 40:95–103
Sharma S, Mohler J, Mahajan SD, Schwartz SA, Bruggemann L, Aalinkeel R (2023) Microbial biofilm: a review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms 11:1614
Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G (2010) Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res 20:510–518
Silva S, Rodrigues CF, Araújo D, Rodrigues ME, Henriques M (2017) Candida species biofilms’ antifungal resistance. J Fungi (basel) 3:8
Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J (2012) Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev 36:288–305
Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, Wu P, Shi Y, Mao F, Yan Y, Xu W, Qian H (2018) Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano 12:7613–7628
Taff HT, Mitchell KF, Edward JA, Andes DR (2013) Mechanisms of Candida biofilm drug resistance. Future Microbiol 8:1325–1337
Talapko J, Juzbašić M, Matijević T, Pustijanac E, Bekić S, Kotris I, Škrlec I (2021) Candida albicans—The virulence factors and clinical manifestations of infection. J Fungi 7:79
Truong T, Zeng G, Qingsong L, Kwang LT, Tong C, Chan FY, Wang Y, Seneviratne CJ (2016) Comparative ploidy proteomics of Candida albicans biofilms unraveled the role of the AHP1 gene in the biofilm persistence against Amphotericin B. Mol Cell Proteomics 15:3488–3500
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8:726–736
Vandenbosch D, Braeckmans K, Nelis HJ, Coenye T (2010) Fungicidal activity of miconazole against Candida spp. biofilms. J Antimicrob Chemother 65:694–700
Wang G, Li X, Wang Z (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44:D1087-1093
Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM (2010) A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 5:e10088
Wong JH, Ng TB, Legowska A, Rolka K, Hui M, Cho CH (2011) Antifungal action of human cathelicidin fragment (LL13-37) on Candida albicans. Peptides 32:1996–2002
Yagi H, Chen AF, Hirsch D, Rothenberg AC, Tan J, Alexander PG, Tuan RS (2020) Antimicrobial activity of mesenchymal stem cells against Staphylococcus aureus. Stem Cell Res Ther 11:293
Yang R, Liu Y, Kelk P, Qu C, Akiyama K, Chen C, Atsuta I, Chen W, Zhou Y, Shi S (2013) A subset of IL-17(+) mesenchymal stem cells possesses anti-Candida albicans effect. Cell Res 23:107–121
Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein RA, Kopf M, Romani L (2007) IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol 37:2695–2706
Zhang B, Tian X, Hao J, Xu G, Zhang W (2020) Mesenchymal stem cell-derived extracellular vesicles in tissue regeneration. Cell Transplant 29:0963689720908500
Zhao AG, Shah K, Cromer B, Sumer H (2020) Mesenchymal stem cell-derived extracellular vesicles and their therapeutic potential. Stem Cells Int 2020:8825771
Zhou W, Xu Y (2020) Chapter 2 - Application of mesenchymal stem cells in human diseases. In: El-Hashash AHK (ed) Mesenchymal Stem Cells in Human Health and Diseases. Academic Press, New York, pp 5–15
Funding
This work received no external funding.
Author information
Authors and Affiliations
Contributions
The author agreed to publish this manuscript in “Archives of Microbiology”. M. Bicer mainly contributed to write this manuscript. The author reviewed the manuscript draft and approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethics approval
The author confirms that this work is original, has not been published elsewhere, and is not currently under consideration for publication elsewhere.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bicer, M. Exploring therapeutic avenues: mesenchymal stem/stromal cells and exosomes in confronting enigmatic biofilm-producing fungi. Arch Microbiol 206, 11 (2024). https://doi.org/10.1007/s00203-023-03744-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03744-0