Abstract
Hip fractures are a global health problem with a high postoperative mortality rate. Preoperative predictors for early mortality could be used to optimise and personalise healthcare strategies. This study aimed to identify predictors for early mortality following hip fracture surgery. Cohort studies examining independent preoperative predictors for mortality following hip fracture surgery were identified through a systematic search on Scopus and PubMed. Predictors for 30-day mortality were the primary outcome, and predictors for mortality within 1 year were secondary outcomes. Primary outcomes were analysed with random-effects meta-analyses. Confidence in the cumulative evidence was assessed using the GRADE criteria. Secondary outcomes were synthesised narratively. Thirty-three cohort studies involving 462,699 patients were meta-analysed. Five high-quality evidence predictors for 30-day mortality were identified: age per year (OR: 1.06, 95% CI: 1.04–1.07), ASA score ≥ 3 (OR: 2.69, 95% CI: 2.12–3.42), male gender (OR: 2.00, 95% CI: 1.85–2.18), institutional residence (OR: 1.81, 95% CI: 1.31–2.49), and metastatic cancer (OR: 2.83, 95% CI: 2.58–3.10). Additionally, six moderate-quality evidence predictors were identified: chronic renal failure, dementia, diabetes, low haemoglobin, heart failures, and a history of any malignancy. Weak evidence was found for non-metastatic cancer. This review found relevant preoperative predictors which could be used to identify patients who are at high risk of 30-day mortality following hip fracture surgery. For some predictors, the prognostic value could be increased by further subcategorising the conditions by severity.
Similar content being viewed by others
Introduction
Hip fractures are a global health problem [1], most commonly affecting adults in their 80s [2]. Due to the increase in life expectancy of the world’s population, an increase in the incidence of hip fractures is expected in the next few years [3, 4]. According to epidemiological projections, 6.26 million individuals will be affected by hip fractures annually by 2050 [5]. Hip fractures are associated with an increased risk of mortality amongst older adults, with a cumulative 30-day mortality rate between 5 and 10% [6]. Over a 1-year postoperative period, it could accumulate up until approximately 30% [4].
In order to identify the patients at greatest risk, preoperative predictors of mortality following hip fracture surgery have been studied extensively [7,8,9]. Predictors for early mortality are particularly important, as they lie at the core of preoperative decision-making in clinical guidelines [10]. Preoperative prognostics could be used to better inform patients and family on the consequences of the different treatment alternatives, leading to better shared decision-making. This is particularly relevant for frail patients with a limited life expectancy, who face higher risk of mortality but not poorer quality of life when they opt for conservative (non-surgical) management following shared decision-making [11]. Therefore, shared decision-making could be used to select a treatment that is optimal in terms of both clinical outcomes and patients’ personal values [12, 13]. During this process, it is essential that decisions are supported by the best available evidence [14]. Meta-analyses can substantiate shared decision-making as they are one of the strongest resources in evidence-based medicine [15].
However, there are several methodological limitations of existing meta-analyses in this field [7,8,9]. Firstly, the effects of predictors have frequently been pooled across widely ranging follow-up times, causing their clinical implication for early mortality to become ambiguous. Secondly, the statistical uncertainty in cumulative evidence, caused by the small number of available studies per predictor, has received little attention so far. Finally, to the best of our knowledge, none of the existing meta-analyses in this field has incorporated the Grading of Recommendation Assessment, Development and Evaluation (GRADE) criteria to assess the confidence in the cumulative evidence per predictor [16, 17]. Therefore, there is ample room for improvement in consolidating the current evidence base.
To support and improve evidence-based medicine for hip fracture patients, it is important to adequately reflect uncertainty in cumulative evidence. This will allow clinicians to assess the risk of early mortality more confidently, helping them to adequately inform their patients. The aim of this study is to conduct a meta-analysis, accompanied by GRADE assessments and sensitivity analyses, to detect valid predictors for early mortality following hip fracture surgery.
Method
This review was reported according to the PRISMA 2020 statement [18].
Search strategy
Scopus and PubMed were searched from inception to 3 November 2021. The search strategy comprised a combination of four key terms relating to older adults, hip fractures, mortality, and predictors. The complete search strategy is shown in Online Resource 1. Additionally, the Dutch Hip Fracture Audit (DHFA) was contacted for internal research reports.
Selection criteria
In this study, the inclusion criteria were as follows: (1) the article describes a cohort study examining preoperative predictors of mortality following hip fracture surgery, (2) the study reports on primary evidence, (3) the article is written in English, and (4) the full-text document can be retrieved. The exclusion criteria were as follows: (1) the article describes an unrepresentative population (i.e. mean/median age below 70 years, solely a single gender included, solely periprosthetic, or pathological fractures included), (2) the article does not report on preoperative predictors, (3) the article does not report on independent risk factors, (4) the article does not report on the statistics of interest (i.e. no odds or hazard ratios and no 95% confidence intervals), (5) the article does not report on mortality as an outcome or reports on mortality as part of a composite score of multiple adverse events, and (6) the article does not report on mortality within 1 year.
Data collection and extraction
The title, abstract, and full-text screenings were performed by MB. The abstract and full-text screenings were independently verified by WSN on a sample basis (70%). Disagreements were resolved through discussion, without need for adjudication by a third reviewer. Study characteristics were extracted onto standardised tables containing author, year, country, study design, sample size, gender distribution, mean/median age, fracture types, treatment types, and mortality rates.
Outcomes
Adjusted odds ratios (ORs) and adjusted hazard ratios (HRs) of preoperative predictors for 30-day mortality following hip fracture surgery were primary outcomes. Independent predictors for mortality within 1 year were secondary outcomes.
Risk of bias
Risk of bias was assessed with the Quality In Prognosis Studies tool [19]. A quarter of the articles were assessed independently by two reviewers (MB, WSN), who collectively refined the protocol to resolve ambiguities in the assessment criteria. The remaining articles were assessed by MB using the refined assessment criteria (Online Resource 2).
Data analysis
All predictors that were reported at least twice were synthesised in narrative summary tables [20], independent of whether they were reported as ORs or HRs. A minimum of three studies was set for quantitative synthesis, and eligibility for pooling was based on consistency in variable definitions. ORs and HRs were meta-analysed separately for each of the predictors, using DerSimonian-Laird random-effects models [21] to accommodate for population and intervention heterogeneity [22,23,24]. Heterogeneity was quantified with the I2 statistic, and results were summarised with forest plots.
Sensitivity analyses were conducted with respect to publication bias and statistical uncertainty caused by the small number of available studies per predictor. The former was inspected with the trim-and-fill method [25] using the \({R}_0^{+}\) and \({L}_0^{+}\) algorithms as recommended by Duval and Tweedie [26]. The latter was inspected with the modified Knapp-Hartung method with ad hoc variance correction [27] and a Bayesian hierarchical model [28] (details on the Bayesian model specification can be found in Online Resource 3). The Bayesian model was particularly of interest for the sensitivity analysis, based on its demonstrated ability to effectively deal with small sample sizes [22, 29,30,31]. All analyses were performed with R version 4.1.2 (R foundation, 2020, Vienna, Austria), using the metafor [32], brms [33], and robvis [34] packages.
Certainty of evidence assessment
Each pooled estimate was appraised using the GRADE criteria [16, 17] (Online Resource 4). When the quality of evidence was inconsistent across multiple pooled estimates of the same predictor, the quality of the pooled estimate based on most studies and patients was chosen for the final appraisal.
Results
Search and included studies
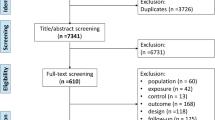
From the initial database yield of 1869 articles, 139 were reviewed in full text after assessing the eligibility based on titles and abstracts. Subsequently, an internal research report published by the DHFA was included and analysed. Reapplication of the exclusion criteria to the full texts yielded 100 articles for narrative synthesis and 33 articles for meta-analysis. The selection process is shown in Fig. 1.
A summary of the characteristics of the included studies is presented in Online Resource 5. Overall, early mortality was studied relatively infrequently: predictors for inpatient mortality were reported in 14 studies [35,36,37,38,39,40,41,42,43,44,45,46,47,48], predictors for 30-day mortality were reported in 35 studies [38, 40, 49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81], and predictors for 1-year mortality were reported in 60 studies. Amongst the 33 studies included in the meta-analysis involving 462,699 patients, one study did not report the 30-day mortality rate [52]. The median 30-day mortality rate and interquartile range across the remaining studies were 8.0% (6.5–9.6%). It was noteworthy that detailed descriptions of the hip fracture aetiology were generally lacking in these studies. The mechanism of injury was only explicitly mentioned in eight studies, with six studies reporting on the exclusion of patients with high-energy trauma [54, 65,66,67, 69, 71] and two studies reporting on the inclusion of them [75, 81]. Similarly, explicit statements on presence of pathological fractures could only be ascertained for 14 studies, with 12 reporting on complete exclusion of them [38, 40, 50, 54, 58, 66, 68,69,70,71, 73, 78] and two studies reporting on the inclusion of them [75, 81].
Risk of bias
Figure 2 depicts the unweighted risk of bias summary of the 33 studies included in the meta-analysis. Twelve articles were judged to be at overall low risk of bias, 15 were judged to have some concerns, and six were judged to be at high risk of bias. High risk was found for bias arising from participation in three studies and for bias arising from confounding in two studies. Amongst the 14 pooled estimates, one had a cumulative weight of high-risk studies of 71.6%. For all remaining pooled estimates, this was below 30% with a median and interquartile range of 2.5% (0–14.0%). The risk of bias assessments of all 100 studies included in the narrative review is shown in Online Resource 6.
Predictors for 30-day mortality
An overview of all meta-analysed predictors for 30-day mortality is shown in Table 1, and forest plots of all high-quality evidence predictors are shown in Fig. 3. The remaining forest plots are shown in Online Resource 7. None of the pooled evidence was downgraded for publication bias based on the outcomes of the sensitivity analysis using the trim-and-fill method.
Forest plots of high-quality evidence predictors for 30-day mortality following hip fracture surgery. The right panel depicts the risk of bias assessments according to the bias domains of the Quality in Prognosis Studies tool, i.e. study participation (D1), study attrition (D2), prognostic factor measurement (D3), outcome measurement (D4), study confounding (D5), and statistical analysis and reporting (D6). The risk of bias levels of low, moderate, and high were colour-coded in green, yellow, and red, respectively
Age
Age was reported as both categorical and continuous variables. Due to inconsistencies in the cut-off levels of age strata [49,50,51, 56, 60, 61, 65, 77], pooling was limited to studies reporting the influence of age per year increase. Analysis of 10 studies [40, 53, 57, 63, 64, 66, 68,69,70, 76] including 154,353 patients provided high-quality evidence that a year increase in age increased the risk of 30-day mortality, with an OR of 1.06 and 95% CI: 1.04–1.07. Figure 3 indicates that the pooled estimate overlapped with all 95% CIs, except for those reported by Cao et al. (1.07–1.08) and Würdemann et al. (1.01–1.05). Since the margin by which the CIs did not overlap was small, the interpretation of I2 was deemed misleading. Therefore, it was decided against downgrading the quality of evidence for inconsistency, despite I2 = 69%.
American Society of Anesthesiologists score
American Society of Anesthesiologists (ASA) scores were reported as both categorical and continuous variables across the studies. Amongst the reports of categorically treated ASA scores, two studies were excluded from pooling as there were insufficient data for the respective cut-off levels [53, 70]. Analysis of six studies [40, 56, 64, 73, 76, 79] including 12,994 patients provided high-quality evidence that individuals in ASA strata III–V were at a greater risk of 30-day mortality than individuals in ASA strata I–II, with an OR of 2.69, 95% CI: 2.12–3.42, and I2 = 0%.
Furthermore, analysis of three studies [57, 63, 78] including 5394 patients provided moderate-quality evidence that each unit increase in ASA score increased the risk of 30-day mortality with an OR of 2.62, 95% CI: 2.21–3.12, and I2 = 0%. The quality of evidence was downgraded by one level for risk of bias as the cumulative weight of studies at high risk of bias was 71.6%.
Chronic renal failure
Renal failure was defined as end-stage renal failure (ESRF) [61], unspecified chronic renal failure (CRF) [70], moderate to severe CRF [75], and a joint stratum of acute renal failure (ARF) and early to end-stage CRF [80]. To keep the analysis homogeneous, instances of ARF were excluded from pooling.
Analysis of three studies [61, 70, 75] including 248,872 patients provided moderate-quality evidence that CRF increased the risk of 30-day mortality, with an OR of 1.61, 95% CI: 1.11–2.34, and I2 = 50%. The quality of evidence was downgraded by one level for imprecision as both the Knapp-Hartung CI (0.52–5.23) and the Bayesian credible interval (CrI) (0.73–3.09) contained the null effect.
Dementia
Three studies did not report their dementia diagnoses [40, 70, 75], three studies reported on dementia in Alzheimer’s disease [52, 77, 80], and one study reported on memory loss, (pre)senile, and vascular dementias [65]. Two studies diagnosed dementia using an Abbreviated Mental Test Score ≤ 6 [49, 67], and one study diagnosed it with a Hodkinson’s Abbreviated Mental Test Score ≤ 6 [82]. Pooled estimates were not stratified by dementia diagnosis.
Analysis of three studies [52, 65, 77] including 29,929 patients provided high-quality evidence that dementia increased the risk of 30-day mortality, with a HR of 1.47, 95% CI: 1.31–1.64, and I2 = 0%.
Furthermore, analysis of seven studies [40, 49, 67, 70, 75, 80, 82] including 389,185 patients provided moderate-quality evidence that dementia increased the risk of 30-day mortality, with an OR of 1.57 and 95% CI: 1.30–1.90. The quality of evidence was downgraded for inconsistency due to substantial heterogeneity (I2 = 94%).
Diabetes
Analysis of four studies [68, 70, 75, 80] including 378,573 patients provided moderate-quality evidence that diabetes increased the risk of 30-day mortality, with an OR of 1.09, 95% CI: 1.01–1.18, and I2 = 28%. The quality of evidence was downgraded for imprecision as both the Knapp-Hartung CI (0.96–1.25) and Bayesian CrI (0.84–1.43) contained the null effect.
Gender
Analysis of 15 studies [38, 40, 49, 51, 53, 56, 57, 63, 68,69,70, 75, 76, 78, 80] including 411,554 patients provided high-quality evidence that males were at a greater risk of 30-day mortality than females, with an OR of 1.99, 95% CI: 1.87–2.13, and I2 = 58%.
Similarly, analysis of six studies [50, 55, 60, 65, 71, 77] including 23,988 patients provided high-quality evidence that males were at a greater risk of 30-day mortality than females, with a HR of 2.13, 95% CI: 1.94–2.34, and I2 = 0%.
Haemoglobin
The influence of haemoglobin (Hb) was tested for anaemia (Hb ≤ 10 g/dL) [49, 55, 67] and per millimole per litre decrease [40, 68, 78]. The former three studies comprised both ORs and HRs, causing an insufficiency in consistent data for pooling.
Analysis of three studies [40, 68, 78] including 5838 patients provided moderate-quality evidence that a millimole per litre decrease in Hb increased the risk of 30-day mortality, with an OR of 1.37, 95% CI: 1.17–1.61, and I2 = 40%. The quality of evidence was downgraded for imprecision as both the Knapp-Hartung CI (0.96–1.96) and Bayesian CrI (0.95–1.94) contained the null effect.
Heart failure
Four studies did not report their heart failure diagnoses [60, 61, 70, 72], two studies diagnosed heart failures using ICD-10 code I50 [75, 80], and one study included multiple hypertensive heart diseases in addition to ICD-10 code I50 [52]. Pooling was limited to studies reporting ORs since there were only two studies reporting HRs [52, 60].
Analysis of five studies [61, 70, 72, 75, 80] including 384,312 patients provided moderate-quality evidence that heart failures increased the risk of 30-day mortality, with an OR of 2.20 and 95% CI: 1.28–3.78. The quality of evidence was downgraded for inconsistency due to substantial heterogeneity (I2 = 99%).
Institutional residence
Analysis of six studies [40, 49, 67, 68, 78, 81] including 12,338 patients provided high-quality evidence that individuals living in an institution were at a greater risk of 30-day mortality than individuals living in their own home, with an OR of 1.81, 95% CI: 1.31–2.49, and I2 = 56%.
Malignancy
Four definitions of malignancies were found: history of any malignancy [61, 68, 80] excluding non-invasive skin cancer [49], non-metastatic cancer [67, 70, 75], and metastatic cancer [70, 72, 75]. Amongst the cases of metastatic cancer, no information could be found on whether bone metastases were included. Separate pooled estimates were computed for a history of any malignancy (excluding non-invasive skin cancer), non-metastatic cancer, and metastatic cancer.
Analysis of four studies [49, 61, 68, 80] including 136,160 patients provided moderate-quality evidence that a history of any malignancy increased the risk of 30-day mortality, with an OR of 2.39 and 95% CI: 1.69–3.38. The quality of evidence was downgraded by one level for inconsistency due to substantial heterogeneity (I2 = 61%).
Furthermore, analysis of three studies [67, 68, 70] including 136,906 patients provided low-quality evidence that non-metastatic cancer increased the risk of 30-day mortality, with an OR of 1.17 and 95% CI: 1.08–1.27. The quality of evidence was downgraded by one level for imprecision as both the Knapp-Hartung CI (0.99–1.73) and Bayesian CrI (0.95–1.86) contained the null effect and by another level for inconsistency due to substantial heterogeneity (I2 = 80%).
Finally, analysis of three studies [70, 72, 80] including 270,355 patients provided high-quality evidence that metastatic cancer increased the risk of 30-day mortality, with an OR of 2.83, 95% CI: 2.58–3.10, and I2 = 0%.
Narrative review findings
The narrative review findings of predictors for postoperative mortality within 1 year, including 30-day mortality, are summarised in Table 2. Overall, the results were congruent with the meta-analysis. For institutional residence, however, the rate at which significant associations with mortality were found differed between short-term and long-term follow-ups. Table 1 shows that two-thirds of the studies contributing to the pooled estimate for institutional residence were insignificant. Upon including 4-month and 1-year follow-ups, two-thirds of the associations tested between institutional residence and mortality were significant.
Discussion
This paper reports on the results of the first GRADE-compliant meta-analysis focusing on predictors of 30-day mortality following hip fracture surgery. In total, five high-quality evidence predictors were identified: age, male gender, ASA classification, institutional residence, and metastatic cancer. Additionally, six moderate-quality evidence predictors were identified: CRF, dementia, diabetes, Hb, heart failures, and a history of any malignancy. Finally, low-quality evidence was found for the influence of non-metastatic cancer.
To optimally use these findings in clinical practice, a few considerations must be made. Firstly, although a history of any malignancy is predictive of 30-day mortality, substantial heterogeneity exists in its prognostic value across studies (I2 = 61%). Mortality risk predictions could be improved if a distinction is made between non-metastatic and metastatic cancer. Our results showed that the respective 95% CIs of 1.11–1.56 and 2.58–3.10 were distinct and showcased little variability, indicating that the mortality risk differed significantly between these two malignancy types. Although the necessity to make this distinction might seem straightforward, various 30-day mortality risk scores have not done this yet [68, 73, 135]. In accordance with the Charlson Comorbidity Index (CCI) [136], risk predictions should distinguish between non-metastatic and metastatic cancer to provide more accurate and personalised prognoses.
Secondly, CRF could manifest itself in different degrees of severity. Amongst the pooled studies, only one exclusively reported on the effect of ESRF [61]. Due to the low ESRF prevalence in 29/746 patients, the respective 95% CI was wide (1.05–10.01). Consequently, the meta-analysis did not reveal a need to stratify the risk estimate by severity of CRF as the individual 95% CIs overlapped by a sufficient margin to keep the between-study heterogeneity within acceptable bounds at I2 = 50%. However, larger studies with ESRF prevalences of 113/3981 [35] and 886/44,419 patients [48] consistently reported larger risks of inpatient mortality with ORs of 6.70 and 95% CI: 4.20–10.69 and 6.70 and 95% CI: 3.57–12.58, respectively. Therefore, the pooled OR of 1.61 reported in this review is unlikely to be representative for patients with ESRF. Especially since CRF is highly prevalent amongst older adults [137], it becomes increasingly important to personalise prognoses based on the severity of CRF, rather than merely its presence or absence.
Thirdly, heart failures might require a more careful definition to be of better prognostic value. The pooled estimate reported in this review exhibited substantial unexplained heterogeneity (I2 = 99%). Even across studies which both resorted to ICD-10 code I50 for heart failure diagnosis [75, 80], the ORs differed substantially (95% CI: 1.54–1.73 vs 95% CI: 3.68–4.13). A disadvantage of ICD-10 code I50 is that it includes both heart failures with preserved ejection fraction and heart failures with reduced ejection fraction. Studies have shown that decreases in the left ventricular ejection fraction (LVEF) generally increase the risk of mortality [138]. Hence, it is postulated that the LVEF is an unobserved variable which could explain the high I2 value. Therefore, future studies should acknowledge the varying degrees of severity in heart failures and report the diagnoses in terms of the LVEF.
Several important limitations are noted. Some studies might have been overlooked since only two databases were searched for this review. Furthermore, the number of studies focusing on independent predictors of 30-day mortality is relatively limited, since most focus on more long-term prognoses. Consequently, the limited number of available studies restricted the use of other diagnostics besides the trim-and-fill method to assess risk of publication bias more reliably. We abstained from using publication bias diagnostics based on funnel plots and Egger’s test due to their very low power [139]. Hence, the conclusions drawn with respect to publication bias should be interpreted with caution.
Furthermore, the list of predictors is incomplete due to restrictions in pooling. Ischaemic heart disease was repeatedly associated with 30-day mortality but could not be pooled as the results were a mix of ORs and HRs [60, 75, 80]. Additionally, inconsistency in reporting was identified as a systemic cause for incompleteness in the list of predictors. The CCI [38, 51, 70] and the number of comorbidities [49, 58, 67] were also repeatedly found to be significant predictors of 30-day mortality. However, they could not be pooled since the cut-off levels by which patients were categorised were inconsistent.
Another issue induced by inconsistency in reporting manifested itself in the quality of pooled evidence. The pooled OR of Hb per millimole per litre decrease was based on three studies instead of five due to inconsistent definitions for the influence of Hb. The respective quality of evidence was now downgraded for imprecision, which is postulated to have arisen due to a lack of power. Had all five studies been eligible for pooling, then sufficient power might have been attained to circumvent downgrading. Hence, future studies should establish which variable definitions and cut-off levels are most clinically relevant to the field of geriatric trauma surgery, e.g. by using the methods reported by Ogawa et al. [140], to improve consistency in reporting.
Conclusion
This study identified five high-quality, six moderate-quality, and one low-quality evidence predictors for 30-day mortality following hip fracture surgery based on preoperative data. Many of the published studies and widely used risk scores define predictors as the mere presence or absence of diseases. To provide better risk predictions, future studies should step away from such coarse definitions. According to the findings in this study, malignancies, CRFs, and heart failures should be further subcategorised by severity to increase their prognostic value in prediction models. Hopefully, the results of this meta-analysis will enable clinicians to better identify patients who are at high risk of 30-day mortality. This information can be used to better inform patients on their prognosis, as one of the contributing factors which may lead to better shared decision-making in the preoperative phase.
References
Griffin XL, Parsons N, Achten J et al (2015) Recovery of health-related quality of life in a United Kingdom hip fracture population. Bone Jt J (97-B):372–382
Haleem S, Lutchman L, Mayahi R et al (2008) Mortality following hip fracture: trends and geographical variations over the last 40 years. Injury 39:1157–1163
Rapp K, Büchele G, Dreinhöfer K et al (2019) Epidemiology of hip fractures. Z Für Gerontol Geriatr 52:10–16
Liu Z, Zhang J, He K et al (2019) Optimized clinical practice for superaged patients with hip fracture: significance of damage control and enhanced recovery program. Burns Trauma 7:21
Kannus P, Parkkari J, Sievänen H et al (1996) Epidemiology of hip fractures. Bone 18:S57–S63
Parker MJ, Palmer CR (1993) A new mobility score for predicting mortality after hip fracture. J Bone Joint Surg Br 75:797–798
Smith T, Pelpola K, Ball M et al (2014) Pre-operative indicators for mortality following hip fracture surgery: a systematic review and meta-analysis. Age Ageing 43:464–471
Chang W, Lv H, Feng C et al (2018) Preventable risk factors of mortality after hip fracture surgery: systematic review and meta-analysis. Int J Surg 52:320–328
Hu F, Jiang C, Shen J et al (2012) Preoperative predictors for mortality following hip fracture surgery: a systematic review and meta-analysis. Injury 43:676–685
Federatie Medisch Specialisten (2019) Richtlijn behandeling kwetsbare ouderen met een proximale femurfractuur. https://richtlijnendatabase.nl/richtlijn/behandeling_kwetsbare_ouderen_bij_chirurgie/generieke_zorgpad.html. Accessed 19 Oct 2023
Loggers SAI, Willems HC, Van Balen R et al (2022) Evaluation of quality of life after nonoperative or operative management of proximal femoral fractures in frail institutionalized patients: the FRAIL-HIP study. JAMA Surg. https://doi.org/10.1001/jamasurg.2022.0089
Stacey D, Légaré F, Lewis K et al (2017) Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001431.pub5
Joosten EA, Defuentes-Merillas L, De Weert GH et al (2008) Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom 77:219–226
Stiggelbout AM, der Weijden TV, Wit MPTD et al (2012) Shared decision making: really putting patients at the centre of healthcare. BMJ 344:e256
Haidich AB (2010) Meta-analysis in medical research. Hippokratia 14:29–37
Foroutan F, Guyatt G, Zuk V et al (2020) GRADE Guidelines 28: use of GRADE for the assessment of evidence about prognostic factors: rating certainty in identification of groups of patients with different absolute risks. J Clin Epidemiol 121:62–70
Iorio A, Spencer FA, Falavigna M et al (2015) Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 350:h870–h870
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Hayden JA, van der Windt DA, Cartwright JL et al (2013) Assessing bias in studies of prognostic factors. Ann Intern Med 158:280–286
Huguet A, Hayden JA, Stinson J et al (2013) Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Syst Rev 2:71
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Borenstein M, Hedges LV, Higgins JPT et al (2009) Introduction to meta-analysis, 1st edn. John Wiley & Sons, Ltd
Borenstein M, Hedges LV, Higgins JPT et al (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1:97–111
Higgins JPT, Thompson SG, Spiegelhalter DJ (2009) A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 172:137–159
Duval S, Tweedie R (2000) A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 95:89–98
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463
Knapp G, Hartung J (2003) Improved tests for a random effects meta-regression with a single covariate. Stat Med 22:2693–2710
Harrer M, Cuijpers P et al (2021) Doing meta-analysis with R: a hands-on guide, 1st edn. Chapman & Hall/CRC Press, Boca Raton, FL and London
Turner RM, Davey J, Clarke MJ et al (2012) Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 41:818–827
Rhodes KM, Turner RM, Higgins JPT (2015) Predictive distributions were developed for the extent of heterogeneity in meta-analyses of continuous outcome data. J Clin Epidemiol 68:52–60
Röver C, Knapp G, Friede T (2015) Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol 15:99
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48
Bürkner P-C (2017) brms: an R package for Bayesian multilevel models using Stan. J Stat Softw 80:1–28
McGuinness LA, Higgins JPT (2021) Risk-of-bias visualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 12:55–61
Jiang HX, Majumdar SR, Dick DA et al (2005) Development and initial validation of a risk score for predicting in-hospital and 1-year mortality in patients with hip fractures. J Bone Miner Res 20:494–500
Myers AH, Robinson EG, Natta MLV et al (1991) Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol 134:1128–1137
Pioli G, Barone A, Giusti A et al (2006) Predictors of mortality after hip fracture: results from 1-year follow-up. Aging Clin Exp Res 18:381–387
Franzo A, Francescutti C, Simon G (2005) Risk factors correlated with post-operative mortality for hip fracture surgery in the elderly: a population-based approach. Eur J Epidemiol 20:985–991
Padrón-Monedero A, López-Cuadrado T, Galán I et al (2017) Effect of comorbidities on the association between age and hospital mortality after fall-related hip fracture in elderly patients. Osteoporos Int 28:1559–1568
Würdemann FS, Wilschut JA, Hegeman JH (2021) Eindverslag SKMS Project Doorontwikkeling DHFA. Dutch Institute for Clinical Auditing
Fisher A, Fisher L, Srikusalanukul W et al (2018) Usefulness of simple biomarkers at admission as independent indicators and predictors of in-hospital mortality in older hip fracture patients. Injury 49:829–840
Ribeiro TA, Premaor MO, Larangeira JA et al (2014) Predictors of hip fracture mortality at a general hospital in South Brazil: an unacceptable surgical delay. Clinics 69:253–258
Tal S, Gurevich A, Sagiv S et al (2016) Predictors of mortality in hip fracture patients. Eur Geriatr Med 7:561–565
Thorne G, Hodgson L (2021) Performance of the Nottingham Hip Fracture Score and Clinical Frailty Scale as predictors of short and long-term outcomes: a dual-centre 3-year observational study of hip fracture patients. J Bone Miner Metab 39:494–500
Eschbach D-A, Oberkircher L, Bliemel C et al (2013) Increased age is not associated with higher incidence of complications, longer stay in acute care hospital and in hospital mortality in geriatric hip fracture patients. Maturitas 74:185–189
Carow J, Carow JB, Coburn M et al (2017) Mortality and cardiorespiratory complications in trochanteric femoral fractures: a ten year retrospective analysis. Int Orthop 41:2371–2380
Sanz-Reig J, Salvador Marín J, Ferrández Martínez J et al (2018) Prognostic factors and predictive model for in-hospital mortality following hip fractures in the elderly. Chin J Traumatol 21:163–169
Belmont PJ, Garcia EJ, Romano D et al (2014) Risk factors for complications and in-hospital mortality following hip fractures: a study using the National Trauma Data Bank. Arch Orthop Trauma Surg 134:597–604
Maxwell MJ, Moran CG, Moppett IK (2008) Development and validation of a preoperative scoring system to predict 30 day mortality in patients undergoing hip fracture surgery. Br J Anaesth 101:511–517
Roche JJW, Wenn RT, Sahota O et al (2005) Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ 331:1374
Kirkland LL, Kashiwagi DT, Burton MC et al (2011) The Charlson Comorbidity Index score as a predictor of 30-day mortality after hip fracture surgery. Am J Med Qual 26:461–467
de Luise C, Brimacombe M, Pedersen L et al (2008) Comorbidity and mortality following hip fracture: a population-based cohort study. Aging Clin Exp Res 20:412–418
Rae HC, Harris IA, McEvoy L et al (2007) Delay to surgery and mortality after hip fracture. ANZ J Surg 77:889–891
Mariconda M, Costa GG, Cerbasi S et al (2015) The determinants of mortality and morbidity during the year following fracture of the hip: a prospective study. Bone Jt J (97-B):383–390
Sheikh HQ, Hossain FS, Aqil A et al (2017) A comprehensive analysis of the causes and predictors of 30-day mortality following hip fracture surgery. Clin Orthop Surg 9:10–18
Chatterton BD, Moores TS, Ahmad S et al (2015) Cause of death and factors associated with early in-hospital mortality after hip fracture. Bone Jt J (97-B):246–251
Thomas CJ, Smith RP, Uzoigwe CE et al (2014) The weekend effect. Bone Jt J (96-B):373–378
Rosso F, Dettoni F, Bonasia DE et al (2016) Prognostic factors for mortality after hip fracture: operation within 48 hours is mandatory. Injury 47(Suppl 4):S91–S97
Aldebeyan S, Nooh A, Aoude A et al (2017) Hypoalbuminaemia-a marker of malnutrition and predictor of postoperative complications and mortality after hip fractures. Injury 48:436–440
Ireland AW, Kelly PJ, Cumming RG (2015) Risk factor profiles for early and delayed mortality after hip fracture: analyses of linked Australian Department of Veterans’ Affairs databases. Injury 46:1028–1035
Karres J, Kieviet N, Eerenberg J-P et al (2018) Predicting early mortality after hip fracture surgery: the hip fracture estimator of mortality Amsterdam. J Orthop Trauma 32:27–33
Lau TW, Fang C, Leung F (2016) Assessment of postoperative short-term and long-term mortality risk in Chinese geriatric patients for hip fracture using the Charlson comorbidity score. Hong Kong Med J Xianggang Yi Xue Za Zhi 22:16–22
Morrissey N, Iliopoulos E, Osmani AW et al (2017) Neck of femur fractures in the elderly: does every hour to surgery count? Injury 48:1155–1158
Nijland LMG, Karres J, Simons AE et al (2017) The weekend effect for hip fracture surgery. Injury 48:1536–1541
Chiu H-C, Chen C-M, Su T-Y et al (2018) Dementia predicted one-year mortality for patients with first hip fracture. Bone Jt J (100-B):1220–1226
Forni C, Gazineo D, D’Alessandro F et al (2019) Predictive factors for thirty day mortality in geriatric patients with hip fractures: a prospective study. Int Orthop 43:275–281
Faizi M, Farrier AJ, Venkatesan M et al (2014) Is body temperature an independent predictor of mortality in hip fracture patients? Injury 45:1942–1945
van de Ree CL, Gosens T, van der Veen AH et al (2020) Development and validation of the Brabant Hip Fracture Score for 30-day and 1-year mortality. HIP Int 30:354–362
Mayordomo-Cava J, Abásolo L, Montero-Fernandez N et al (2020) Hip fracture in nonagenarians: characteristics and factors related to 30-day mortality in 1177 patients. J Arthroplasty 35:1186–1193
Cao Y, Forssten MP, Mohammad Ismail A et al (2021) Predictive values of preoperative characteristics for 30-day mortality in traumatic hip fracture patients. J Pers Med 11:353
Wang Z, Chen X, Yang L et al (2021) A new preoperative risk score for predicting mortality of elderly hip fracture patients: an external validation study. Aging Clin Exp Res 33:2519–2527
Crawford ZT, Southam B, Matar R et al (2020) A nomogram for predicting 30-day mortality in elderly patients undergoing hemiarthroplasty for femoral neck fractures. Geriatr Orthop Surg Rehabil 11:2151459320960087
Nijmeijer WS, Folbert EC, Vermeer M et al (2016) Prediction of early mortality following hip fracture surgery in frail elderly: the Almelo Hip Fracture Score (AHFS). Injury 47:2138–2143
Norring-Agerskov D, Madsen CM, Abrahamsen B et al (2017) Hyperkalemia is associated with increased 30-day mortality in hip fracture patients. Calcif Tissue Int 101:9–16
Norring-Agerskov D, Madsen CM, Bathum L et al (2019) History of cardiovascular disease and cardiovascular biomarkers are associated with 30-day mortality in patients with hip fracture. Osteoporos Int 30:1767–1778
Pang C, Aqil A, Mannan A et al (2020) Hip fracture patients admitted to hospital on weekends are not at increased risk of 30-day mortality as compared with weekdays. J Orthop Traumatol 21:23
Petersen JD, Siersma VD, Wehberg S et al (2020) Clinical management of hip fractures in elderly patients with dementia and postoperative 30-day mortality: a population-based cohort study. Brain Behav 10:e01823
Schuijt HJ, Bos J, Smeeing DPJ et al (2021) Predictors of 30-day mortality in orthogeriatric fracture patients aged 85 years or above admitted from the emergency department. Eur J Trauma Emerg Surg 47:817–823
Foss NB, Kehlet H (2006) Short-term mortality in hip fracture patients admitted during weekends and holidays. Br J Anaesth 96:450–454
Bottle A, Aylin P (2006) Mortality associated with delay in operation after hip fracture: observational study. BMJ 332:947–951
Khan MA, Hossain FS, Ahmed I et al (2013) Predictors of early mortality after hip fracture surgery. Int Orthop 37:2119–2124
Lizaur-Utrilla A, Gonzalez-Navarro B, Vizcaya-Moreno MF et al (2019) Altered seric levels of albumin, sodium and parathyroid hormone may predict early mortality following hip fracture surgery in elderly. Int Orthop 43:2825–2829
Aharonoff GB, Koval KJ, Skovron ML et al (1997) Hip fractures in the elderly: predictors of one year mortality. J Orthop Trauma 11:162–165
Adunsky A, Arad M, Koren-Morag N et al (2012) Increased 1-year mortality rates among elderly hip fracture patients with atrial fibrillation. Aging Clin Exp Res 24:233–238
Ariza-Vega P, Kristensen MT, Martín-Martín L et al (2015) Predictors of long-term mortality in older people with hip fracture. Arch Phys Med Rehabil 96:1215–1221
Bellelli G, Mazzola P, Corsi M et al (2012) The combined effect of ADL impairment and delay in time from fracture to surgery on 12-month mortality: an observational study in orthogeriatric patients. J Am Med Dir Assoc 13:664.e9–664.e14
Björkelund KB, Hommel A, Thorngren K-G et al (2009) Factors at admission associated with 4 months outcome in elderly patients with hip fracture. AANA J 77:49–58
Bokshan SL, Marcaccio SE, Blood TD et al (2018) Factors influencing survival following hip fracture among octogenarians and nonagenarians in the United States. Injury 49:685–690
Cenzer IS, Tang V, Boscardin WJ et al (2016) One-year mortality after hip fracture: development and validation of a prognostic index. J Am Geriatr Soc 64:1863–1868
Elliott J, Beringer T, Kee F et al (2003) Predicting survival after treatment for fracture of the proximal femur and the effect of delays to surgery. J Clin Epidemiol 56:788–795
Endo Y, Aharonoff GB, Zuckerman JD et al (2005) Gender differences in patients with hip fracture: a greater risk of morbidity and mortality in men. J Orthop Trauma 19:29–35
Flodin L, Laurin A, Lökk J et al (2016) Increased 1-year survival and discharge to independent living in overweight hip fracture patients: a prospective study of 843 patients. Acta Orthop 87:146–151
Folbert EC, Hegeman JH, Vermeer M et al (2017) Improved 1-year mortality in elderly patients with a hip fracture following integrated orthogeriatric treatment. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 28:269–277
Giummarra MJ, Ekegren CL, Gong J et al (2020) Twelve month mortality rates and independent living in people aged 65 years or older after isolated hip fracture: a prospective registry-based study. Injury 51:420–428
Henderson CY, Ryan JP (2015) Predicting mortality following hip fracture: an analysis of comorbidities and complications. Ir J Med Sci 184:667–671
Ho C-A, Li C-Y, Hsieh K-S et al (2010) Factors determining the 1-year survival after operated hip fracture: a hospital-based analysis. J Orthop Sci Off J Jpn Orthop Assoc 15:30–37
Huette P, Abou-Arab O, Djebara A-E et al (2020) Risk factors and mortality of patients undergoing hip fracture surgery: a one-year follow-up study. Sci Rep 10:9607
Hung L-W, Hwang Y-T, Huang G-S et al (2017) The influence of renal dialysis and hip fracture sites on the 10-year mortality of elderly hip fracture patients: a nationwide population-based observational study. Medicine (Baltimore) 96:e7618
Kang H-Y, Yang K, Kim YN et al (2010) Incidence and mortality of hip fracture among the elderly population in South Korea: a population-based study using the national health insurance claims data. BMC Public Health 10:230
Kim S-M, Moon Y-W, Lim S-J et al (2012) Prediction of survival, second fracture, and functional recovery following the first hip fracture surgery in elderly patients. Bone 50:1343–1350
Mazzola P, Bellelli G, Broggini V et al (2015) Postoperative delirium and pre-fracture disability predict 6-month mortality among the oldest old hip fracture patients. Aging Clin Exp Res 27:53–60
Menéndez-Colino R, Alarcon T, Gotor P et al (2018) Baseline and pre-operative 1-year mortality risk factors in a cohort of 509 hip fracture patients consecutively admitted to a co-managed orthogeriatric unit (FONDA Cohort). Injury 49:656–661
Meng D, Bai X, Wu H et al (2021) Patient and perioperative factors influencing the functional outcomes and mortality in elderly hip fractures. J Investig Surg Off J Acad Surg Res 34:262–269
Nuotio M, Tuominen P, Luukkaala T (2016) Association of nutritional status as measured by the Mini-Nutritional Assessment Short Form with changes in mobility, institutionalization and death after hip fracture. Eur J Clin Nutr 70:393–398
O’Daly BJ, Walsh JC, Quinlan JF et al (2010) Serum albumin and total lymphocyte count as predictors of outcome in hip fractures. Clin Nutr Edinb Scotl 29:89–93
Pereira SRM, Puts MTE, Portela MC et al (2010) The impact of prefracture and hip fracture characteristics on mortality in older persons in Brazil. Clin Orthop 468:1869–1883
Petersen MB, Jørgensen HL, Hansen K et al (2006) Factors affecting postoperative mortality of patients with displaced femoral neck fracture. Injury 37:705–711
Söderqvist A, Ekström W, Ponzer S et al (2009) Prediction of mortality in elderly patients with hip fractures: a two-year prospective study of 1,944 patients. Gerontology 55:496–504
Talsnes O, Hjelmstedt F, Dahl OE et al (2011) Clinical and biochemical prediction of early fatal outcome following hip fracture in the elderly. Int Orthop 35:903–907
Vosoughi AR, Emami MJ, Pourabbas B et al (2017) Factors increasing mortality of the elderly following hip fracture surgery: role of body mass index, age, and smoking. Musculoskelet Surg 101:25–29
Xing F, Luo R, Chen W et al (2021) The risk-adjusted Charlson Comorbidity Index as a new predictor of one-year mortality rate in elderly Chinese patients who underwent hip fracture surgery. Orthop Traumatol Surg Res OTSR 107:102860
Yombi JC, Putineanu DC, Cornu O et al (2019) Low haemoglobin at admission is associated with mortality after hip fractures in elderly patients. Bone Jt J (101-B):1122–1128
Baidoo PK, Odei JB, Ansu V et al (2021) Predictors of hip fracture mortality in Ghana: a single-center prospective study. Arch Osteoporos 16:35
Camur S, Celik H (2019) Prediction of the mortality with comorbidity - polypharmacy score in the osteoporotic hip fractures. Acta Chir Orthop Traumatol Cech 86:320–323
Chen Y-P, Kuo Y-J, Liu C et al (2021) Prognostic factors for 1-year functional outcome, quality of life, care demands, and mortality after surgery in Taiwanese geriatric patients with a hip fracture: a prospective cohort study. Ther Adv Musculoskelet Dis 13:1759720X211028360
Fu G, Li M, Xue Y et al (2021) Rapid preoperative predicting tools for 1-year mortality and walking ability of Asian elderly femoral neck fracture patients who planned for hip arthroplasty. J Orthop Surg 16:455
Yoo JI, Kim H, Ha YC et al (2018) Osteosarcopenia in patients with hip fracture is related with high mortality. J Korean Med Sci 33:e27
Mangoni AA, van Munster BC, Woodman RJ et al (2013) Measures of anticholinergic drug exposure, serum anticholinergic activity, and all-cause postdischarge mortality in older hospitalized patients with hip fractures. Am J Geriatr Psychiatry 21:785–793
Söderqvist A, Miedel R, Ponzer S et al (2006) The influence of cognitive function on outcome after a hip fracture. J Bone Joint Surg Am 88:2115–2123
Bell JJ, Pulle RC, Crouch AM et al (2016) Impact of malnutrition on 12-month mortality following acute hip fracture. ANZ J Surg 86:157–161
Bliemel C, Sielski R, Doering B et al (2016) Pre-fracture quality of life predicts 1-year survival in elderly patients with hip fracture-development of a new scoring system. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 27:1979–1987
Ishidou Y, Koriyama C, Kakoi H et al (2017) Predictive factors of mortality and deterioration in performance of activities of daily living after hip fracture surgery in Kagoshima. Japan. Geriatr Gerontol Int 17:391–401
Kannegaard PN, van der Mark S, Eiken P et al (2010) Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing 39:203–209
Heyes GJ, Tucker A, Marley D et al (2017) Predictors for 1-year mortality following hip fracture: a retrospective review of 465 consecutive patients. Eur J Trauma Emerg Surg Off Publ Eur Trauma Soc 43:113–119
Lizaur-Utrilla A, Martinez-Mendez D, Collados-Maestre I et al (2016) Early surgery within 2 days for hip fracture is not reliable as healthcare quality indicator. Injury 47:1530–1535
Kim B-G, Lee Y-K, Park H-P et al (2016) C-reactive protein is an independent predictor for 1-year mortality in elderly patients undergoing hip fracture surgery: a retrospective analysis. Medicine (Baltimore) 95:e5152
Camurcu Y, Cobden A, Sofu H et al (2017) What are the determinants of mortality after cemented bipolar hemiarthroplasty for unstable intertrochanteric fractures in elderly patients? J Arthroplasty 32:3038–3043
Härstedt M, Rogmark C, Sutton R et al (2015) Impact of comorbidity on 6-month hospital readmission and mortality after hip fracture surgery. Injury 46:713–718
Zanetti M, Gortan Cappellari G, Ratti C et al (2019) Poor nutritional status but not cognitive or functional impairment per se independently predict 1 year mortality in elderly patients with hip-fracture. Clin Nutr Edinb Scotl 38:1607–1612
Wu L-C, Chou M-Y, Liang C-K et al (2016) Factors affecting one-year mortality of elderly patients after surgery for hip fracture. Int J Gerontol 10:207–211
D’Angelo F, Giudici M, Molina M et al (2005) Mortality rate after hip hemiarthroplasty: analysis of risk factors in 299 consecutives cases. J Orthop Traumatol 6:111–116
Velez M, Palacios-Barahona U, Paredes-Laverde M et al (2020) Factors associated with mortality due to trochanteric fracture. A cross-sectional study. Orthop Traumatol Surg Res 106:135–139
Kovar FM, Endler G, Wagner OF et al (2015) Basal haemoglobin levels as prognostic factor for early death in elderly patients with a hip fracture--a twenty year observation study. Injury 46:1018–1022
Lawrence JE, Fountain DM, Cundall-Curry DJ et al (2017) Do patients taking warfarin experience delays to theatre, longer hospital stay, and poorer survival after hip fracture? Clin Orthop 475:273–279
Moppett IK, Parker M, Griffiths R et al (2012) Nottingham Hip Fracture Score: longitudinal and multi-centre assessment. Br J Anaesth 109:546–550
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Stevens LA, Viswanathan G, Weiner DE (2010) Chronic kidney disease and end-stage renal disease in the elderly population: current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis 17:293–301
Sweitzer NK, Lopatin M, Yancy CW et al (2008) Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol 101:1151–1156
Simmonds M (2015) Quantifying the risk of error when interpreting funnel plots. Syst Rev 4:24
Ogawa T, Schermann H, Kobayashi H et al (2021) Age and clinical outcomes after hip fracture surgery: do octogenarian, nonagenarian and centenarian classifications matter? Age Ageing 50:1952–1960
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bui, M., Nijmeijer, W.S., Hegeman, J.H. et al. Systematic review and meta-analysis of preoperative predictors for early mortality following hip fracture surgery. Osteoporos Int 35, 561–574 (2024). https://doi.org/10.1007/s00198-023-06942-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06942-0