Abstract
Summary
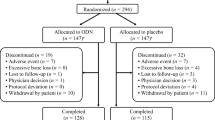
The efficacy and safety of oral placebo or odanacatib 10, 25, or 50 mg once weekly for 52 weeks were evaluated in a double-blind, randomized, multi-center study in Japanese female and male patients with osteoporosis.
Introduction
Odanacatib is a selective and reversible cathepsin K inhibitor that decreases bone resorption and increases bone mineral density (BMD).
Methods
The primary efficacy endpoint was percent change from baseline to week 52 in lumbar spine BMD. Secondary endpoints included percent change in total hip, femoral neck, and trochanter BMD and in bone biomarkers after treatment for 52 weeks.
Results
In this study, 286 patients [94 % female, mean age (SD) 68.2 (7.1) years] were included in the analysis. The least-squares mean percent changes from baseline to week 52 in the groups receiving placebo, 10, 25 and 50 mg of odanacatib for lumbar spine (L1∼L4) BMD were 0.5, 4.1, 5.7, and 5.9 % and for total hip BMD were −0.4, 1.3, 1.8, and 2.7 %, respectively. The changes in femoral neck and trochanter BMD were similar to those at the total hip. Bone turnover markers were reduced in a dose-dependent manner. However, the effects of odanacatib on bone formation markers were less compared with the effects on bone resorption markers. Tolerability and safety profiles were similar among all treatment groups with no dose-related trends in any adverse events.
Conclusions
Weekly odanacatib treatment for 52 weeks increased BMD at the lumbar spine and at all hip sites in a dose-dependent manner and was well tolerated in Japanese patients with osteoporosis.




Similar content being viewed by others
References
Consensus Development Conference (1993) Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94:646–650
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporosis fractures. Osteoporos Int 17:1726–1733
Report of a WHO scientific group (2003) Prevention and management of osteoporosis. 2003 WHO Technical Report Series 921:1–164
Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, Soen S, Nishizawa Y, Hagino H, Fukunaga M, and Fujiwara S (2012) Japanese 2011 guidelines for prevention and treatment of osteoporosis—executive summary. Arch Osteoporos. 7:3–20 of Osteoporosis 2011 Edition. Lifescience, p4. (in Japanese)
Fujiwara S, Kasagi F, Masunari N, Naito K, Suzuki G, Fukunaga M (2003) Fracture prediction from bone mineral density in Japanese men and women. J Bone Miner Res 18:1547–1553
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199
Wehren LE, Hosking D, Hochberg MC (2004) Putting evidence-based medicine into clinical practice: comparing anti-resorptive agents for the treatment of osteoporosis. Curr Med Res Opin 20:525–531
Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK (2006) Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 355:125–137
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Trovas GP, Lyritis GP, Galanos A, Raptou P, Constantelou E (2002) A randomized trial of nasal spray salmon calcitonin in men with idiopathic osteoporosis: effects on bone mineral density and bone markers. J Bone Miner Res 17:521–527
Cryer B, Bauer DC (2002) Oral bisphosphonates and upper gastrointestinal tract problems: what is the evidence? Mayo Clin Proc 77:1031–1043
Watts NB, Diab DL (2010) Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab 95:1555–1565
Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster D, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Koval K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Sen HT, van der Meulen MCH, Weinstein RS, Whyte M (2010) Atypical subtrochanteric and diaphyseal femoral fractures. Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 25:2267–2294
Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B (2009) American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg 67:2–12
Yasuda Y, Kaleta J, Brömme D (2005) The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev 57:973–993
Vasiljeva O, Reinheckel T, Peters C, Turk D, Turk V, Turk B (2007) Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr Pharm Des 13:387–403
Stoch SA, Wagner JA (2008) Cathepsin K inhibitors: a novel target for osteoporosis therapy. Clin Pharmacol Ther 83:172–176
Gauthier JY, Chauret N, Cromlish W, Desmarais S, le Duong T, Falgueyret JP, Kimmel DB, Lamontagne S, Léger S, LeRiche T, Li CS, Massé F, McKay DJ, Nicoll-Griffith DA, Oballa RM, Palmer JT, Percival MD, Riendeau D, Robichaud J, Rodan GA, Rodan SB, Seto C, Thérien M, Truong VL, Venuti MC, Wesolowski G, Young RN, Zamboni R, Black WC (2008) The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett 18:923–928
Stoch S, Zajic S, Stone J, Miller D, Van Dyck K, Gutierrez M, De Decker M, Liu L, Liu Q, Scott B, Panebianco D, Jin B, Duong L, Gottesdiener K, Wagner J (2009) Effect of the cathepsin K inhibitor odanacatib on bone resorption biomarkers in healthy postmenopausal women: two double-blind, randomized, placebo-controlled phase I studies. Clin Pharmacol Ther 86:175–182
Leung P, Pickarski M, Zhuo Y, Masarachia PJ, Duong LT (2011) The effects of the cathepsin K inhibitor odanacatib on osteoclastic bone resorption and vesicular trafficking. Bone 49:623–635
Bone HG, McClung MR, Roux C, Recker RR, Eisman JA, Verbruggen N, Hustad CM, Dasilva C, Santora AC, Ince BA (2010) Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res 25:937–947
Eisman JA, Bone HG, Hosking DJ, McClung MR, Reid IR, Rizzoli R, Resch H, Verbruggen N, Hustad CM, DaSilva C, Petrovic R, Santora AC, Ince BA, Lombardi A (2011) Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res 26:242–251
Langdahl B, Binkley N, Bone H, Gilchrist N, Resch H, Portales JR, Denker A, Lombardi A, De Tilleghem CL, Dasilva C, Rosenberg E, Leung A (2012) Odanacatib in the treatment of postmenopausal women with low bone mineral density: 5 years of continued therapy in a phase 2 study. J Bone Miner Res 27:2251–2258
Orimo H, Sugioka Y, Fukunaga M, Muto Y, Hotokebuchi T, Gorai I, Nakamura T, Kushida K, Tanaka H, Ikai T, Oh-hashi Y, The Committee of the Japanese Society for Bone and Mineral Research for Development of Diagnostic Criteria of Osteoporosis (1998) Diagnostic criteria of primary osteoporosis. J Bone Miner Metab 16:139–150
Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H, Osteoporosis Diagnostic Criteria Review Committee: Japanese Society for Bone and Mineral Research (2001) Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab 19:331–337
Uchida S, Shiraki M, Fukunaga M, Tomomitsu T, Fujimoto G, Nakagomi M, Leung A, Zajic S, Santora A, Stone J, Passarell J, Nakamura T (2012) The results of a double-blind randomized phase 2 dose-finding study of odanacatib, a potent cathepsin K inhibitor, in Japanese patients with osteoporosis with a model-based pharmacokinetic (PK) analysis. J Bone Miner Res 27 (Suppl 1). Available at http://www.asbmr.org/Meetings/AnnualMeeting/Abstracts12.apx. Accessed 29 January 2013
Uchida S, Taniguchi T, Shimizu T, Kakikawa T, Okuyama K, Okaniwa M, Arizono H, Nagata K, Santora AC, Shiraki M, Fukunaga M, Tomomitsu T, Ohashi Y, Nakamura T (2005) Therapeutic effects of alendronate 35 mg once weekly and 5 mg once daily in Japanese patients with osteoporosis: a double-blind, randomized study. J Bone Miner Metab 23:382–388
Bonnick S, Saag KG, Kiel DP, McClung M, Hochberg M, Burnett SM, Sebba A, Kagan R, Chen E, Thompson DE, de Papp AE (2006) Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab 91:2631–2637
Reid DM, Hosking D, Kendler D, Brandi ML, Wark JD, Marques-Neto JF, Weryha G, Vgrbruggen N, Hustad CM, Mahlis EM, Melton ME (2008) A comparison of the effect of alendronate and risedronate on bone mineral density in postmenopausal women with osteoporosis: 24-month results from FACTS-international. Int J Clin Pract 62:575–584
Schnitzer T, Bone HG, Crepaldi G, Adami S, McClung M, Kiel D, Felsenberg D, Recker RR, Tonino RP, Roux C, Pinchera A, Foldes AJ, Greenspan SL, Levine MA, Emkey R, Santora AC 2nd, Kaur A, Thompson DE, Yates J, Orloff JJ (2000) Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Aging Clin Exp Res 12:1–12
Acknowledgments
We thank Drs. Albert T. Leung, Antonio Lombardi, and Keith Kaufman, Merck Research Laboratories, Rahway, New Jersey, for their kind comments and advice on the manuscript. We are also indebted to Mr. Jun Matsumoto, MSD.K.K, for his scientific and technical support. The following primary investigators and clinical sites in Japan participated in this study: T. Okano, Tottori University Hospital; T. Yamaguchi, Shimane University Hospital; H. Fukunishi, Shinsuma General Hospital; R. Takayanagi, Kyushu University Hospital; S. Okamoto, SORF Okamoto Clinic; K. Shigenobu, Hakodate Central General Hospital; T. Ohta, Ohta Orthopaedic Clinic; M. Takahashi, Takahashi Orthopedics Clinic; T. Oguma, Sapporo Orthopaedic Hospital; S. Mori, Mori’s clinic of orthopedic rheumatic; S. Nakajo, Nakajo Orthopedic Clinic; E. Nakamura, University of Occupational and Environmental Health Japan; S. Okamoto, KS Okamoto Clinic; K. Kuroda, Kuroda Orthopedic Clinic; T. Okada, Okada Orthopedic Clinic; Y. Nakatani, Nakatani Hospital; T. Higuchi, Hirosaki University School of Medicine & Hospital; K. Sugiyasu, Kirishima Sugiyasu Hospital; A. Tomonaga, Tana Orthopedics Clinic; K. Watanabe, Kitashinyokohama Orthopedic Clinic; H. Yamane, Toyooka Daiichi Hospital; K. Kinoshita, Seijo Kinoshita Hospital; K. Akazawa, Akazawa Ladies’ Clinic; Y. Fujii, Fujii Medical Clinic; Y. Miki, Medical Juridical Person Sanpoukai Nanko-Hospital; H. Kaneko, National Hospital Organization Saitama National Hospital; S. Ichikawa, Cardiovascular Hospital of Central Japan; Y. Somekawa, Toride Kyodo General Hospital; T. Hanaoka, Yamabeonsen Hanaoka Orthopedic Clinic; M. Fujimori, Keyakidori Orthopedics; H. Hanashi, New Medical Research System Clinic; R. Suto, Yokohama Shin-Midori General Hospital; K. Sakamoto, Nishikamata Seikeigeka; H. Tanaka, Moji Rosai Hospital; H. Miyajima, Meguro You + I Clinic; K. Sato, Nagoya Daini Red Cross Hospital; S. Muraki, The University of Tokyo Hospital; H. Takagi, Nagoya Kyouritsu Clinic; H. Shimizu, Shimizu Orthopedic Clinic; T. Mori, Sapporo Tokushukai medical center; A. Itabashi, Kubojima Clinic; H. Ito, Ito Orthopedic Surgery Clinic.
Conflicts of interest
Authors Santora, Tsai, Fujimoto, Nakagomi, Tsubouchi, E. Rosenberg, and Uchida are employed by, and may hold stock options with, Merck Sharp & Dohme, a subsidiary of Merck & Co. Inc. Nakamura has received research grants/consulting fees from Merck. Shiraki reports consultant/speaker’s bureau fees from Merck. The remaining authors report no financial relationships with Merck Sharp & Dohme, the sponsor of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakamura, T., Shiraki, M., Fukunaga, M. et al. Effect of the cathepsin K inhibitor odanacatib administered once weekly on bone mineral density in Japanese patients with osteoporosis—a double-blind, randomized, dose-finding study. Osteoporos Int 25, 367–376 (2014). https://doi.org/10.1007/s00198-013-2398-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2398-2