Abstract
In the field of topology optimization for fluid flow design, there is a specific class of formulations directed to non-Newtonian fluids. One prominent case is when the fluid is blood. In this case, in addition to the non-Newtonian effect, the damage caused by the flow to the blood (i.e., blood damage) should be taken into account. Blood damage is essentially given by two types: hemolysis, which corresponds to the breakdown of Red Blood Cells (RBCs), and thrombosis, which corresponds to the formation of blood clotting. More specifically, in terms of thrombosis, blood clotting is formed by the aggregation of platelets and RBCs in vessels. Furthermore, in order to model thrombosis, there are essentially two approaches—platelet activation (initiation of thrombosis) and platelet aggregation. However, the computational cost of the second approach is still considered impractical for real applications. In the topology optimization field, hemolysis and thrombosis have been normally assumed to be indirectly minimized by considering the shear stress (or even energy dissipation or vorticity). However, a recent work has considered the direct minimization of hemolysis from a differential equation model. In terms of thrombosis, the stress levels for damage are 10 times lower than hemolysis, which means that it may not be sufficient to consider only the minimization of hemolysis in the design. Therefore, in this work, the topology optimization is formulated in order to take thrombosis into account, computed by a platelet activation model. The resulting formulation is also set to consider hemolysis and relative energy dissipation (as a way to indirectly maximize efficiency). In terms of thrombosis, the shear-induced platelet activation model is here rewritten for a finite elements approach, while also considering the necessary adjustments for the topology optimization formulation. The topology optimization is also formulated for a non-Newtonian fluid model for blood, and the optimization solver is IPOPT (Interior Point Optimization algorithm). Some numerical examples are presented considering 2D swirl flow configurations and a 2D configuration.




























Similar content being viewed by others
References
Abraham F, Behr M, Heinkenschloss M (2005) Shape optimization in steady blood flow: a numerical study of non-Newtonian effects. Comput Methods Biomech Biomed Engineering 8(2):127–137
Alemu Y, Bluestein D (2007) Flow-induced platelet activation and damage accumulation in a mechanical heart valve: numerical studies. Artif Organs 31(9):677–688
Alexandersen J, Andreasen CS (2020) A review of topology optimisation for fluid-based problems. Fluids 5(1):29
Alonso DH, Silva ECN (2021) Topology optimization for blood flow considering a hemolysis model. Struct Multidisc Optim 63(5):2101–2123. https://doi.org/10.1007/s00158-020-02806-x
Alonso DH, de Sá LFN, Saenz JSR, Silva ECN (2019) Topology optimization based on a two-dimensional swirl flow model of tesla-type pump devices. Comput Math Appl 77(9):2499–2533. https://doi.org/10.1016/j.camwa.2018.12.035
Alonso DH, Saenz JSR, Silva ECN (2020) Non-Newtonian laminar 2d swirl flow design by the topology optimization method. Struct Multidisc Optim 62(1):299–321. https://doi.org/10.1007/s00158-020-02499-2
Amestoy PR, Duff IS, Koster J, L’Excellent JY (2001) A fully asynchronous multifrontal solver using distributed dynamic scheduling. SIAM J Matrix Anal Appl 23(1):15–41
Antaki JF, Ghattas O, Burgreen GW, He B (1995) Computational flow optimization of rotary blood pump components. Artif Organs 19(7):608–615
Apel J, Paul R, Klaus S, Siess T, Reul H (2001) Assessment of hemolysis related quantities in a microaxial blood pump by computational fluid dynamics. Artif Organs 25(5):341–347. https://doi.org/10.1046/j.1525-1594.2001.025005341.x
Arora D, Behr M, Pasquali M (2004) A tensor-based measure for estimating blood damage. Artif Organs 28(11):1002–1015
Arora D, Behr M, Pasquali M (2012) Errata. Artif Organs 36(5):500–500. https://doi.org/10.1111/j.1525-1594.2012.01491.x
Bagot CN, Arya R (2008) Virchow and his triad: a question of attribution. Br J Haematol 143(2):180–190. https://doi.org/10.1111/j.1365-2141.2008.07323.x
Barthes-Biesel D, Rallison J (1981) The time-dependent deformation of a capsule freely suspended in a linear shear flow. J Fluid Mech 113:251–267
Behbahani M, Behr M, Hormes M, Steinseifer U, Arora D, CORONADO O, Pasquali M (2009) A review of computational fluid dynamics analysis of blood pumps. Eur J Appl Math 20:363–397
Bird RB, Armstrong RC, Hassager O (1987) Dynamics of polymeric liquids, volume 1: fluid mechanics, 1st edn. Wiley, Hoboken
Borrvall T, Petersson J (2003) Topology optimization of fluids in stokes flow. Int J Numer Methods Fluids 41(1):77–107. https://doi.org/10.1002/fld.426
Brass LF (2003) Thrombin and platelet activation. Chest 124(3):18S-25S
Cheng R, Lai YG, Chandran KB (2004) Three-dimensional fluid-structure interaction simulation of bileaflet mechanical heart valve flow dynamics. Ann Biomed Eng 32(11):1471–1483
Cho YI, Kenssey KR (1991) Effects of the non-Newtonian viscosity of blood on flows in a diseased arterial vessel. Part 1: Steady flows. Biorheology 28:241–262
Chopard B, de Sousa DR, Lätt J, Mountrakis L, Dubois F, Yourassowsky C, Van Antwerpen P, Eker O, Vanhamme L, Perez-Morga D, Courbebaisse G, Lorenz E, Hoekstra AG, Boudjeltia KZ (2017) A physical description of the adhesion and aggregation of platelets. R Soc Open Sci 4(4):170219
Consolo F, Valerio L, Brizzola S, Rota P, Marazzato G, Vincoli V, Reggiani S, Redaelli A, Fiore G (2016) On the use of the platelet activity state assay for the in vitro quantification of platelet activation in blood recirculating devices for extracorporeal circulation. Artif Organs 40(10):971–980
Ding J, Chen Z, Niu S, Zhang J, Mondal NK, Griffith BP, Wu ZJ (2015) Quantification of shear-induced platelet activation: high shear stresses for short exposure time. Artif Organs 39(7):576–583
Dodsworth L (2016) Operational parametric study of a prototype tesla pump. Master’s thesis, Dalhousie University
Dorman FD, Murphy TE, Blackshear PL (1966) An application of the tesla viscous flow turbine to pumping blood: mechanical devices to assist the failing heart. In: National research council. National Academy of Science, pp 119–128
Farinas MI, Garon A, Lacasse D, N’dri D (2006) Asymptotically consistent numerical approximation of hemolysis. J Biomech Eng 128(5):688–696. https://doi.org/10.1115/1.2241663
Farrell PE, Ham DA, Funke SW, Rognes ME (2013) Automated derivation of the adjoint of high-level transient finite element programs. SIAM J Sci Comput 35(4):C369–C393
Forchheimer P (1901) Wasserbewegung durch boden. Z Ver Deutsch, Ing 45:1782–1788
Fraser K, Taskin M, Zhang T, Griffith B, Wu Z (2010) Comparison of shear stress, residence time and lagrangian estimates of hemolysis in different ventricular assist devices. In: 26th southern biomedical engineering conference SBEC 2010, April 30–May 2, 2010. College Park, Maryland, USA, Springer, pp 548–551
Garon A, Farinas MI (2004) Fast three-dimensional numerical hemolysis approximation. Artif Organs 28(11):1016–1025
Ghattas O, He B, Antaki JF (1995) Shape optimization of Navier-Stokes flows with application to optimal design of artificial heart components. Tech. rep, Carnegie Institute of Technology, Department of Civil and Environmental Engineering
Giersiepen M, Wurzinger L, Opitz R, Reul H (1990) Estimation of shear stress-related blood damage in heart valve prostheses-in vitro comparison of 25 aortic valves. Int J Artif Organs 13(5):300–306
Gijsen FJH, van de Vosse FN, Janssen JD (1999) The influence of the non-Newtonian properties of blood on the flow in large arteries: steady flow in a carotid bifurcation model. J Biomech 32(6):601–608. https://doi.org/10.1016/S0021-9290(99)00015-9
Grigioni M, Morbiducci U, D’Avenio G, Di Benedetto G, Del Gaudio C (2005) A novel formulation for blood trauma prediction by a modified power-law mathematical model. Biomech Model Mechanobiol 4(4):249–260
Gurtin ME (1981) An introduction to continuum mechanics, 1st edn. Academic Press, New York
Hansen KB, Arzani A, Shadden SC (2015) Mechanical platelet activation potential in abdominal aortic aneurysms. J Biomech Eng 137(4):041005
Hasinger SH, Kehrt LG (1963) Investigation of a shear-force pump. J Eng Power 85(3):201–206
Hellums JD (1994) 1993 Whitaker lecture: biorheology in thrombosis research. Ann Biomed Eng 22(5):445–455
Hinghofer-Szalkay H, Greenleaf J (1987) Continuous monitoring of blood volume changes in humans. J Appl Physiol 63(3):1003–1007
Hyun J, Wang S, Yang S (2014) Topology optimization of the shear thinning non-Newtonian fluidic systems for minimizing wall shear stress. Comput Math Appl 67(5):1154–1170. https://doi.org/10.1016/j.camwa.2013.12.013
Izraelev V, Weiss WJ, Fritz B, Newswanger RK, Paterson EG, Snyder A, Medvitz RB, Cysyk J, Pae WE, Hicks D, Lukic B, Rosenberg G (2009) A passively-suspended tesla pump left ventricular assist device. ASAIO J (American Society for Artificial Internal Organs: 1992) 55(6):556
Jensen KE (2013) Structural optimization of non-newtonian microfluidics. PhD thesis, Technical University of Denmark, phD thesis
Jiang L, Chen S, Sadasivan C, Jiao X (2017) Structural topology optimization for generative design of personalized aneurysm implants: design, additive manufacturing, and experimental validation. In: 2017 IEEE healthcare innovations and point of care technologies (HI-POCT), IEEE, pp 9–13
Kian JM (2017) Topology optimization method applied to design channels considering non-Newtonian fluid flow. Master’s thesis, Universidade de São Paulo, http://www.teses.usp.br/teses/disponiveis/3/3152/tde-16032017-103709/en.php
Kini V, Bachmann C, Fontaine A, Deutsch S, Tarbell J (2001) Integrating particle image velocimetry and laser doppler velocimetry measurements of the regurgitant flow field past mechanical heart valves. Artif Organs 25(2):136–145
Lai WM, Rubin DH, Krempl E, Rubin D (2009) Introduction to continuum mechanics. Butterworth-Heinemann, Oxford
Lazarov BS, Sigmund O (2010) Filters in topology optimization based on Helmholtz-type differential equations. Int J Numer Methods Eng 86(6):765–781
Leondes C (2000) Biomechanical systems: techniques and applications, volume II: cardiovascular techniques, biomechanical systems: techniques and applications, 1st edn. CRC Press, Boca Raton
Logg A, Mardal KA, Wells G (2012) Automated solution of differential equations by the finite element method: the FEniCS book, vol 84. Springer. https://fenicsproject.org/book/
Mitusch S, Funke S, Dokken J (2019) dolfin-adjoint 2018.1: automated adjoints for fenics and firedrake. J Open Source Software 4(38):1292https://doi.org/10.21105/joss.01292
Monroe DM, Hoffman M, Roberts HR (2002) Platelets and thrombin generation. Arterioscler Thromb Vasc Biol 22(9):1381–1389
Montevecchi F, Inzoli F, Redaelli A, Mammana M (1995) Preliminary design and optimization of an ECC blood pump by means of a parametric approach. Artif Organs 19(7):685–690
Munson BR, Young DF, Okiishi TH (2009) Fundamentals of fluid mechanics, 6th edn. Wiley, Hoboken
Nam J, Behr M, Pasquali M (2011) Space-time least-squares finite element method for convection-reaction system with transformed variables. Comput Methods Appl Mech Eng 200(33–36):2562–2576
Nobili M, Sheriff J, Morbiducci U, Redaelli A, Bluestein D (2008) Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO J (American Society for Artificial Internal Organs: 1992) 54(1):64
Packham MA (1994) Role of platelets in thrombosis and hemostasis. Can J Physiol Pharmacol 72(3):278–284
Pauli L, Nam J, Pasquali M, Behr M (2013) Transient stress-based and strain-based hemolysis estimation in a simplified blood pump. Int J Numer Methods Biomed Eng 29(10):1148–1160
Philippi B, Jin Y (2015) Topology optimization of turbulent fluid flow with a sensitive porosity adjoint method (spam). arXiv:1512.08445
Pratumwal Y, Limtrakarn W, Muengtaweepongsa S, Phakdeesan P, Duangburong S, Eiamaram P, Intharakham K (2017) Whole blood viscosity modeling using power law, Casson, and Carreau Vasuda models integrated with image scanning u-tube viscometer technique. Songklanakarin J Sci Technol 39(5):625–631
Reddy JN, Gartling DK (2010) The finite element method in heat transfer and fluid dynamics, 3rd edn. CRC Press, Boca Raton
Rey Ladino AF (2004) Numerical simulation of the flow field in a friction-type turbine (tesla turbine). Diploma thesis, Institute of Thermal Powerplants, Vienna University of Technology
Romero J, Silva E (2014) A topology optimization approach applied to laminar flow machine rotor design. Comput Methods Appl Mech Eng 279(Supplement C):268–300. https://doi.org/10.1016/j.cma.2014.06.029
Romero JS, Silva ECN (2017) Non-Newtonian laminar flow machine rotor design by using topology optimization. Struct Multidisc Optim 55(5):1711–1732
Sabersky RH, Acosta AJ, Hauptmann EG, Gates EM (1971) Fluid flow: a first course in fluid mechanics, vol 299. Macmillan, London
Sheriff J, Bluestein D, Girdhar G, Jesty J (2010) High-shear stress sensitizes platelets to subsequent low-shear conditions. Ann Biomed Eng 38(4):1442–1450
Sheriff J, JaS Soares, Xenos M, Jesty J, Bluestein D (2013) Evaluation of shear-induced platelet activation models under constant and dynamic shear stress loading conditions relevant to devices. Ann Biomed Eng 41(6):1279–1296
Soares JS, Sheriff J, Bluestein D (2013) A novel mathematical model of activation and sensitization of platelets subjected to dynamic stress histories. Biomech Model Mechanobiol 12(6):1127–1141
Sonntag RE, Borgnakke C (2013) Fundamentals of thermodynamics, 8th edn. Wiley, Hoboken
Tesch K (2013) On invariants of fluid mechanics tensors. Task Q 17(3–4):228–230
Travis BR, Marzec UM, Leo HL, Momin T, Sanders C, Hanson SR, Yoganathan AP (2001) Bileaflet aortic valve prosthesis pivot geometry influences platelet secretion and anionic phospholipid exposure. Ann Biomed Eng 29(8):657–664
Wächter A, Biegler LT (2006) On the implementation of an interior-point filter line-search algorithm for large-scale nonlinear programming. Math Program 106(1):25–57
Ward JC (1964) Turbulent flow in porous media. J Hydraul Div 90(5):1–12
White FM (2009) Fluid mechanics, 7th edn. McGraw-Hill, New York, p 2011
Wu J (2007) Letter to the editor: A possible major mistake in the paper entitled “collected nondimensional performance of rotary dynamic blood pump”: Smith WA, Allaire P, Antaki J, Butler KC, Kerkhoffs W, Kink T, Loree H, Reul H. Asaio J 53(2):255–256
Wu J, Antaki JF, Snyder TA, Wagner WR, Borovetz HS, Paden BE (2005) Design optimization of blood shearing instrument by computational fluid dynamics. Artif Organs 29(6):482–489
Yu H (2015) Flow design optimization of blood pumps considering hemolysis. PhD thesis, Magdeburg, Universität, Diss., 2015
Yun SH, Sim EH, Goh RY, Park JI, Han JY (2016) Platelet activation: the mechanisms and potential biomarkers. BioMed Res Int. https://doi.org/10.1155/2016/9060143
Zhang B, Liu X (2015) Topology optimization study of arterial bypass configurations using the level set method. Struct Multidisc Optim 51(3):773–798. https://doi.org/10.1007/s00158-014-1175-y
Zhang B, Liu X, Sun J (2016) Topology optimization design of non-Newtonian roller-type viscous micropumps. Struct Multidisc Optim 53(3):409–424
Funding
This research was partly supported by CNPq (Brazilian National Research Council) and FAPESP (São Paulo Research Foundation). The authors thank the supporting institutions. The first author thanks the financial support of FAPESP under Grant 2017/27049-0. The second author thanks the financial support of CNPq (National Council for Research and Development) under Grant 302658/2018-1 and of FAPESP under Grant 2013/24434-0. The authors also acknowledge the support of the RCGI (Research Centre for Gas Innovation), which is hosted by the University of São Paulo (USP) and sponsored by FAPESP (2014/50279-4) and Shell Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Replication of results
The descriptions of the formulation, the numerical implementation and the numerical results contain all the necessary information for reproducing the results of this article. Also, the tutorials/examples present in http://www.dolfin-adjoint.org may help with the implementation.
Additional information
Responsible Editor: Nestor V. Queipo
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A: Finite element formulation for the fluid flow problem
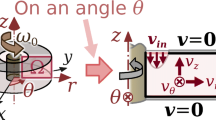
In order to define the finite element formulation, it is necessary to first define the corresponding boundary value problem. By considering the types of computational domains illustrated in Fig. 29, (Alonso et al. 2019)
Figure 29 shows the interior of the computational domain (\({\Omega }\)), and the boundaries (\({\Gamma }_{\text {in}}\), \({\Gamma }_{\text {wall}}\), \({\Gamma }_{\text {out}}\), and \({\Gamma }_{\text {sym}}\)). On the inlet boundary (\({\Gamma }_{\text {in}}\)), the velocity profile is fixed, while, on the walls (\({\Gamma }_{\text {wall}}\)), the no-slip condition is set. On the outlet boundary (\({\Gamma }_{\text {out}}\)), the stress free Neumann boundary condition is set, while \({\varvec{n}}\) is the unit vector that is normal to the boundaries—i.e., pointing outside the computational domain, and being given, in the 2D swirl flow model, as \({\varvec{n}} = (n_r,\ 0,\ n_z)\), and, in the 2D model, as \({\varvec{n}} = (n_x,\ n_y)\). On the symmetry axis (\({\Gamma }_{\text {sym}}\), used in one of the 2D swirl flow cases in Fig. 29), there is a symmetry axis boundary condition: the derivatives in relation to the r coordinate are zero, and the radial velocity is also zero. The stress tensor (\({\varvec{T}}\)) is indicated as \({\varvec{T}}(\mu ({\dot{\gamma }}_{\text {m}}))\), when considering the fluid being modeled from the non-Newtonian fluid model, in Eq. (6).
From the formulation presented in Eq. (52), the finite element method is considered to solve the equilibrium equations for the 2D swirl flow model, by considering the weighted-residual and the Galerkin methods for the velocity-pressure (mixed) formulation, (Reddy and Gartling 2010; Alonso and Silva 2021)

where the equations are indicated by subscripts: c (denoting the continuity equation), and m (denoting the linear momentum equation—i.e., the Navier-Stokes equations). The corresponding test functions are indicated as: \({{w}}_{p}\) (pressure test function), and \({{{\varvec{w}}}}_{v} =\left[ \begin{matrix}{{w}}_{v,r}\\ {{w}}_{v,\theta }\\ {{w}}_{v,z}\end{matrix}\right]\) (velocity test function). Since the integration domain (\(2\pi r d{\Omega }\)) includes a constant multiplier (\(2\pi\)), which exerts no influence when solving the weak form, Eqs. (53) and (54) are given including a division by \(2\pi\) (Alonso et al. 2019).
In the case of a 2D model, the integration domain is different (i.e., in Cartesian coordinates), meaning that, in Eqs. (53) and (54), \(rd{\Omega }\) should be replaced by \(d{\Omega }\), while \(rd{\Gamma }\) should be replaced by \(d{\Gamma }\).
The test functions (\({{w}}_{p}\) and \({{{\varvec{w}}}}_{v}\)) in Eqs. (53) and (54) are mutually independent. This means that the corresponding equations may be summed, resulting in a single equation
Appendix B: LSFEM formulation for the thrombosis model
The resulting LSFEM (Least Squares Finite Element Method) formulation from Eqs. (27), (28), (29) and (30) becomes:
where \({{w}}_{S_{\tau }}\), \({{w}}_{D_{PAS,sl}}\), \({{w}}_{D_{PAS,sr}}\) and \({{w}}_{I_{\text{PAS}}}\) are the test functions.
Notice that \((\varvec{v}{\cdot }\nabla )D_{PAS,sl}\) and \((\varvec{v}{\cdot }\nabla )D_{PAS,sr}\) in Eq. (30) can be substituted from Eqs. (28) and (29), but the resulting system of equations would achieve a worse numerical conditioning (as has been observed in some tests). The numerical conditioning was observed to be much better when using the formulation presented in Eq. (30).
Rights and permissions
About this article
Cite this article
Alonso, D.H., Silva, E.C.N. Blood flow topology optimization considering a thrombosis model. Struct Multidisc Optim 65, 179 (2022). https://doi.org/10.1007/s00158-022-03251-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00158-022-03251-8