Abstract
Purpose
The use of arterial partial pressure of carbon dioxide (PaCO2) as a target intervention to manage elevated intracranial pressure (ICP) and its effect on clinical outcomes remain unclear. We aimed to describe targets for PaCO2 in acute brain injured (ABI) patients and assess the occurrence of abnormal PaCO2 values during the first week in the intensive care unit (ICU). The secondary aim was to assess the association of PaCO2 with in-hospital mortality.
Methods
We carried out a secondary analysis of a multicenter prospective observational study involving adult invasively ventilated patients with traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), intracranial hemorrhage (ICH), or ischemic stroke (IS). PaCO2 was collected on day 1, 3, and 7 from ICU admission. Normocapnia was defined as PaCO2 > 35 and to 45 mmHg; mild hypocapnia as 32–35 mmHg; severe hypocapnia as 26–31 mmHg, forced hypocapnia as < 26 mmHg, and hypercapnia as > 45 mmHg.
Results
1476 patients (65.9% male, mean age 52 \(\pm\) 18 years) were included. On ICU admission, 804 (54.5%) patients were normocapnic (incidence 1.37 episodes per person/day during ICU stay), and 125 (8.5%) and 334 (22.6%) were mild or severe hypocapnic (0.52 and 0.25 episodes/day). Forced hypocapnia and hypercapnia were used in 40 (2.7%) and 173 (11.7%) patients. PaCO2 had a U-shape relationship with in-hospital mortality with only severe hypocapnia and hypercapnia being associated with increased probability of in-hospital mortality (omnibus p value = 0.0009). Important differences were observed across different subgroups of ABI patients.
Conclusions
Normocapnia and mild hypocapnia are common in ABI patients and do not affect patients' outcome. Extreme derangements of PaCO2 values were significantly associated with increased in-hospital mortality.
Similar content being viewed by others
Extreme values of hypo- and hypercapnia are associated with in-hospital mortality in acute brain injured patients, with mild hypocapnia being better tolerated, especially in the traumatic brain injured subpopulation. The targets of carbon dioxide should take in consideration the type of acute brain injury as well as physiological/neuromonitoring data. |
Introduction
Changes in the arterial partial pressure of carbon dioxide (PaCO2) can exert profound effects on cerebrovascular regulation, leading to adjustments in cerebrovascular tone, cerebral blood flow (CBF), and cerebral blood volume (CBV) in acute brain-injured (ABI) patients [1, 2]. Induced hypocapnia secondary to hyperventilation is utilized as a secondary intervention to control elevated intracranial pressure (ICP) that remains unresponsive to initial therapeutic measures [3,4,5]. Nevertheless, it is essential to exercise caution when employing induced hypocapnia, as some studies have demonstrated to cause cerebral hypoperfusion [1], particularly when reaching PaCO2 levels < 25 mmHg.
Indeed, prophylactic or prolonged use of extreme hypocapnia is discouraged based on the most recent recommendations from the Brain Trauma Foundation Guidelines, supported by level IIB evidence [6], and it should be considered only in cases of life-threatening brain herniation. The most recent evidence-based Guidelines of the European Society of Intensive Care Medicine (ESICM) on ventilatory management of ABI patients have not provided definitive recommendations regarding target PaCO2 levels in case of intracranial hypertension [7]. The lack of conclusive guidance is attributed to the limited quality of evidence available on this complex pathophysiological framework. Therefore, the optimal cutoffs for PaCO2 in acute brain-injured patients, with and without intracranial hypertension, are currently under debate.
We performed a secondary analysis of a worldwide multicenter prospective observational study conducted in ABI patients who received invasive ventilation in the intensive care unit (ICU), with the aim to assess the incidence of abnormal PaCO2 values on admission and during the first week of ICU stay. We also assessed the association between PaCO2 values and in-hospital mortality in the entire population, as well as in different ABI subgroups.
Methods
Study design and ethics
This is a preplanned secondary analysis of a worldwide, multicenter, observational cohort study named “Extubation strategies in Neuro-Intensive care unit patients and associations with Outcomes” (ENIO) (registration number NCT03400904) conducted between June 2018 and November 2020 [8]. ENIO was endorsed by the PROtective VEntilation Network (PROVE Net), the ESICM, the French Society of Anesthesiology and Critical care (SFAR), and Colegio Mexicano de Medicina Critica (CMMC). The study protocol was approved by the International Review Board (IRB) of the Groupe Nantais d’Éthique dans le Domaine de la Santé, Nantes, France (IRB No. 7/11/2017), and then by each participating site. Informed consent was collected in accordance with the local regulations. Initial approval also included the secondary analysis, which underwent a preliminary evaluation and was approved by the Steering Committee of ENIO. This study was conducted according to the STROBE reporting guidelines for observational studies [9] (electronic supplementary material, ESM, Table S1).
Patients
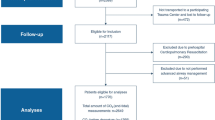
Inclusion criteria of the ENIO study were: (1) age ≥ 18 years; (2) patients receiving invasive ventilation ≥ 24 h; (3) admitted for ABI (including traumatic brain injury—TBI; subarachnoid hemorrhage—SAH; intracranial hemorrhage—ICH; ischemic stroke—IS) with a Glasgow Coma Scale (GCS) score ≤ 12; (4) having underwent an attempt to discontinue ventilation (defined as an extubation trial and/or tracheostomy). Pregnant patients, patients with spinal cord injury above T4, post-cardiac arrest patients, and Guillain–Barré syndrome patients were excluded. ENIO also excluded patients with withdrawal of life-sustaining treatment (WLST) in the first 24 h of ICU admission, patients with tracheostomy before ICU admission, and patients with major respiratory comorbidities or major chest trauma [10]. For this subanalysis, we additionally excluded patients for whom PaCO2 data were missing at admission.
Management of acute brain injury
The primary care of brain damage, according to the main ENIO study, was specific to the initial pathology (trauma, subarachnoid hemorrhage, intracranial bleeding, etc.) and followed international guidelines [3, 5] as well as local practice. Neuromonitoring, brain imaging, and surgical approach were carried out in accordance with local procedures of each center [10].
Data collection
Data extracted from the ENIO dataset included: demographic and baseline characteristics [age, gender, height, weight, body mass index (BMI), previous comorbidities (chronic obstructive pulmonary disease, cardiovascular comorbidities, arterial hypertension, smoking, diabetes mellitus, history of malignancy, etc.)]; type and severity of brain injury (as for GCS); neurosurgical and neurocritical care management, including tier three therapies (barbiturate coma, therapeutic hypothermia, ICP monitoring, and decompressive craniectomy) according to the Seattle Guidelines [3, 5]; airway and ventilatory management (i.e., tracheostomy, gag reflex, cough, spontaneous breathing trial, extubation, and reintubation); in-ICU events, in-hospital mortality, and in-ICU outcomes (need for and duration of IMV, ICU length of stay [LOS], mortality, need for non-invasive mechanical ventilation, and duration).
Definitions
Patients were initially binned in five PaCO2 groups, according to the values on day 1 at ICU admission, according to previous literature [11] and on most recent guidelines [3, 5] as follows: normocapnia, PaCO2 > 35–45 mmHg, mild hypocapnia 32–35 mmHg, severe hypocapnia as 26– < 32 mmHg, forced hypocapnia as PaCO2 < 26 mmHg, and hypercapnia as PaCO2 > 45 mmHg. Abnormal PaCO2 was defined as a value < 35 mmHg and > 45 mmHg. Samples of PaCO2 were obtained in the morning, at around 8 am, as for ENIO protocol.
Objectives
The primary objective was to describe the PaCO2 values measured in ABI patients and assess the occurrence of abnormal PaCO2 values on admission and during the ICU stay in the study cohort. The secondary objective was to assess the association between PaCO2 values and in-hospital mortality in the entire population and according to different brain injury groups (i.e., TBI, SAH, ICH, and IS).
Statistical analysis
Continuous variables are reported as mean ± standard deviation (SD) or median (interquartile range [IQR]) as appropriate; discrete variables are presented as numbers and percentages. For continuous baseline characteristics, ANOVA was used for comparing PaCO2 discrete categories (forced, severe, and mild hypocapnia, normocapnia, and hypercapnia); if the variable did not meet the normality assumption, Kruskal–Wallis test was used instead. For the comparison of discrete variables, a Chi-square test was used.
For descriptive purposes, PaCO2 continuous (PaCO2c) was log-transformed due to high skewness. As such, its trajectory over days 1, 3, and 7 was depicted using a locally weighted scatterplot smoothing (LOWESS) method [12]. We used longitudinal survival regression for inferential analysis. Using this method, we directly evaluated the association between PaCO2 (continuous and categorized) and in-hospital mortality. For this analysis, PaCO2c was log-transformed due to high skewness. The model assumed a Weibull distribution for the baseline hazard and random intercept on patient identification (ID). Variable selection was carried out by backward elimination using a multivariable fractional polynomial (FP) procedure (see ESM for further details) [13]. The baseline variables selected (at day 1) by this method included: age (years), gender, history of hypertension, diabetes, and heart failure, treatment with midazolam, neuromuscular blockers, pentothal, propofol, and dexmedetomidine, ventilatory mode, respiratory rate (breaths/min), tidal volume (mL/kg of predicted body weight [PBW]), positive end-expiratory pressure (PEEP), arterial pH (pHa), GCS, and acute respiratory distress syndrome (ARDS). Results from the model of PaCO2 with 5 discrete categories were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). Based on the U-shaped association suggested by the PaCO2 categorical model, we decided to model PaCO2c with 5-df restricted cubic splines (RCS) to capture the non-linear trajectory [14]. Within this framework, the result was instead depicted through a graph where the HR on the y-scale was plotted against the continuum of PaCO2c. To account for interdependence among centers, the models included a cluster-based adjustment of the standard error estimation.
As a second step, we used a linear mixed-effects model. We evaluated the differences in the log of PaCO2c over the 3 days (using day 1 as reference); the model included a random intercept on the study center and on patient ID to account for correlated measurements from the two clusters. In addition, the variable ICU days (1, 3, and 7) was included as a random coefficient with an unstructured covariance matrix. This model also included the same subset of covariates used in the longitudinal survival regression. Results were expressed as predicted means—also known as least square means (LSM).
Relative distribution analysis [15] evaluated the association between the continuum of PaCO2c and the cumulative proportion of in-hospital mortality with the results depicted as a figure, where the vertical axis shows the relative density of the two distributions (as a ratio) and the horizontal axis shows the percentiles of the reference group (PaCO2c values when in-hospital mortality = 0). Further details on statistical analysis are provided in ESM, Item S1. A significance level < 0.05 was used for all analyses. Stata 18.0 was used for data clean-up and preparation.
Results
Patients
Of the 1512 patients enrolled in the ENIO study, a total of 1476 were included in the analysis (36 patients were excluded for missing PaCO2 data). The mean age of the study cohort was 52 (\(\pm\) 18) years, and 973 (65.9%) were male. Mean BMI was 26.3 (\(\pm\) 5.1) kg/m2; 715 (48.4%) were admitted for TBI, 264 (17.9%) for SAH, 509 (34.5%) for ICH, and 132 (9.2%) for IS (Table 1 and Table S2 for additional data). Demographic characteristics, ventilator management, and arterial blood gas parameters according to neurologic status (GCS > 9 and ≤ 9), type of ABI, and different categories of PaCO2 are presented in ESM, Tables S3–S9.
Carbon dioxide values on admission and during ICU stay
On admission, median PaCO2 was 37 (34–41) mmHg; 804 (54.5%) patients presented with normocapnia on ICU admission, 125 (8.5%) patients were hypercapnic, and 334 (22.6%) and 173 (11.7%) were mild and moderate hypocapnic; forced hypocapnia was observed in 40 (2.7%) patients (Table 1). Significant differences were observed among different PaCO2 categories according to the country of admission, gender, and type of ABI (Table 1, and ESM, Tables S2–S9). Normo- and mild hypocapnia were more frequently observed in the presence of ICP monitoring (p < 0.001), but not in patients who received tier three therapies or according to GCS and pupils’ characteristics (Table 1). The occurrence of abnormal PaCO2 values over time is shown in Fig. 1; only a minority experienced multiple events of abnormal PaCO2 values, with mild hypocapnia being more common than other abnormalities.
Number of patients experiencing abnormally low and high partial pressure of carbon dioxide-PaCO2-episodes during the ICU stay. The bars indicate the number of patients with 0, 1, 2, and 3 episodes of abnormal PaCO2. Incidence rates (as number of episodes per 1 person-day) of hypocapnia and hypercapnia during ICU stay are provided. IR, incidence rate; CI, Confidence Interval
The median PaCO2 significantly increased over time in the whole population from day 1 to 3 (from 37 mmHg [34–41] to 38 mmHg [36–37]; p < 0.001) and from day 1 to 7 (from 37 mmHg [34–41] to 39 mmHg [39–40], p < 0.001) in the whole population (ESM, Figure S2) and in all ABI categories (except ischemic stroke, ESM, Figure S4). Figure 2 and supplemental Figure S3 present the transition of the different PaCO2 categories from day 1 to day 3 and day 7. Most of the changes occurring in the hypocapnia group were toward normocapnia, and only a few were toward severe hypocapnia. Most patients with forced hypocapnia remained in the same category. Most patients with hypercapnia evolved toward normocapnia.
Sankey plot providing a visual representation of the transition of patients with different categories of abnormal PaCO2 at day 1, 3, and 7. The different colors show the number of patients in each PaCO2 category and how they changed from day 1 to day 7. Most of the transitions occurring in the hypocapnia group were toward normocapnia. Most patients with forced hypocapnia remained in the same category, whereas most patients with hypercapnia evolved toward normocapnia. Overall, the pattern of flows between days 3 and 7 was similar to the pattern of flows between days 1 and 3
Effect of carbon dioxide on in-hospital mortality in the overall cohort
The overall in-hospital mortality in the population was 12.1% (n = 178 patients); trajectories of PaCO2 values during ICU stay in survivors vs non-survivors are presented in supplemental Figures S6–S8. When modeled as a continuous variable, PaCO2 had a U-shaped relationship with in-hospital mortality, with both hypo- and hypercapnia being independently associated with an increased probability of in-hospital mortality (omnibus p = 0.0009—Fig. 3a).
A Association between PaCO2 (modeled as a continuous variable) and in-hospital mortality using a longitudinal survival regression. PaCO2c was modeled with 5-df restricted cubic splines (RCS). For esthetic purposes, PaCO2 distribution was trimmed between 20 mmHg and 60 mmHg. The graph shows the adjusted hazard ratio (aHR) for in-hospital mortality on the y-axis across the full range of PaCO2 values on the x-axis. The solid line represents the hazard ratio and the shaded area is the 95% confidence interval. B The association between categorical PaCO2 values and in-hospital mortality risk using a longitudinal survival regression. The graph shows the hazard ratios with 95% confidence intervals using normocapnia a reference category
When PaCO2 was modeled according to its discrete categories, the U-shaped curve persisted (Fig. 3b). Compared to normocapnia, patients with forced hypocapnia (adjusted hazard ratio [aHR] 4.31; 95% CI 1.56–11.87—p = 0.005) had the highest rate of in-hospital mortality, followed by hypercapnia (aHR 3.67; 95% CI = 1.75–7.71—p = 0.001), and severe hypocapnia (aHR 2.77; 95% CI 1.31–5.87—p = 0.008). Patients with mild hypocapnia had a borderline significant association with increased in-hospital mortality (aHR 2.07; 95% CI 1.02–4.12—p = 0.049).
Relative distribution analysis further confirmed the U-shaped association between hypo- and hypercapnia and in-hospital mortality (Fig. 4). In particular, a significant increase in the probability for in-hospital mortality for values > 45 mmHg and < 32 mmHg. Similar results were confirmed when PaCO2 values were assessed separately on each day (Fig. 4b).
a and b Relative distribution analysis aimed at exploring the relationship between the entire distribution of partial pressure of carbon dioxide, PaCO2c with in-hospital mortality. The horizontal axis shows the cumulative proportion of the reference group (PaCO2c values when in-hospital mortality = 0). The vertical axis shows the relative density, which is the ratio of densities between the two distributions at each percentile of the reference group. The 95% confidence intervals indicate whether the relative density at each percentile is significantly different from 1
The relationship between PaCO2 stratified according to neurologic status (GCS > 9 and ≤ 9) and according to the single time points of observation showed a consistent U-shaped curve (supplemental Figures S1 and S9).
Effect of carbon dioxide on in-hospital mortality in different ABI subgroups’ population
In TBI patients (supplemental Figures S10 and S11), a statistically significant association with mortality was found only for severe hypocapnia (aHR 4.43; 95% CI 1.29–15.21—p = 0.018) and hypercapnia (aHR 4.58; 95% CI 1.81–11.58—p = 0.001), but not for other PaCO2 categories. In patients with ICH (supplemental Figures S12 and S13), only hypercapnia was significantly associated with increased mortality (aHR 3.47; 95% CI 1.08–11.18—p = 0.037).
In patients with SAH (Figures S14 and S15), forced hypocapnia (aHR 46.76; 95% CI = 7.22–302.69—p < 0.001), severe (aHR = 7.04; 95% CI = 2.12–23.42—p = 0.001), and mild hypocapnia (HR 3.68; 95% CI = 1.12–12.05—p = 0.032) were independently associated with in-hospital mortality, whereas hypercapnia was not. In patients with IS (supplemental Figures S16 and S17), any of the PaCO2 categories were significantly associated with in-hospital mortality, as compared with normocapnia.
Discussion
In this worldwide multicenter observational cohort of severe ABI patients, we found that—in the early days of ICU admission—PaCO2 values were generally maintained within normal to mild hypocapnia ranges, and that both profound hypo- and hypercapnia were associated with mortality. However, the prevalence and the effect of PaCO2 on in-hospital mortality importantly change according to the type of ABI, with mild hypocapnia being better tolerated in the TBI and ICH population, when compared to the SAH and IS.
Current European Guidelines on mechanical ventilation in ABI patients are inconclusive regarding the optimal target of PaCO2 [7]; the Seattle algorithm suggests maintaining normocapnia as the first instance, and then target to a value of 35–38 mmHg as tier 1 and eventually 32–35 mmHg (mild hypocapnia) as tier 2, avoiding profound hypocapnia except for life-threatening conditions and risk of brain herniation. Although low PaCO2 values can lead to a reduction in ICP, and possibly improve cerebral autoregulation, the consequent vasoconstriction related to hypocapnia can potentially lead to a transitory reduction of CBF and oxygenation [16,17,18].
Some authors demonstrated an increase of cerebral hypoperfused areas using positron emission tomography (PET) following hyperventilation [19], as well as important changes in cerebral metabolism or microdialysis data, suggesting a higher risk of cerebral ischemia [20, 21]. However, other studies [22, 23] suggested that mild and short-term hypocapnia can be safe on cerebral function, causing minimal and not clinically important changes in brain oxygenation and metabolism [23, 24].
The only randomized-controlled trial on this topic [25] was performed on a small group of patients more than 30 years ago, explored the effect of prophylactic hypocapnia, and the results were inconclusive because of important methodological limitations. A recent CENTER-TBI study [4] suggested that large variability across countries still exists regarding PaCO2 targets, but clinicians tend to use mean values of PaCO2 of 38.9 (SD ± 5.2) mmHg, with mean minimum PaCO2 of 35.2 (SD ± 5.3) mmHg, and even lower values are generally used in patients receiving ICP and having intracranial hypertension.
Our study confirms these results regarding the use of relatively low values of PaCO2 adopted in the clinical practice, as in the whole population the median value was 37 mmHg on day 1, and only slightly increased over the following days. Mild hypocapnia was commonly observed and was more frequently used when an ICP probe was inserted and in TBI patients vs. other pathologies. This suggests that clinicians tend to target PaCO2 to a normo-/mild hypocapnia in ABI and especially in TBI patients where a higher number of evidence/recommendations are available, and especially in the early phase of ICU admission when patients have more important issues in controlling ICP.
Interestingly, in the above-mentioned CENTER-TBI study, centers where profound hyperventilation was used did not present worsened outcomes [4]. On the other hand, a recent study from the BRAIN-PROTECT group [26], including a cohort of 1776 TBI patients with end-tidal (ET) CO2 levels measured during prehospital care, found a L-shaped association between ETCO2 levels and 30-day mortality, with important increase in mortality for values below 35 mmHg. However, this study presents important limitations, as it refers only to a limited timeframe setting (prehospital) and does not totally take in account confounding physiological factors which can have influenced decreases in ETCO2. Our results are importantly different; we found a clear U-shaped curve in the whole population, with a steeper part of the curve for hypercapnia, and a tendency to a better outcome for normocapnia or mild hypocapnia. Interestingly, this curve had different shapes when comparing different types of ABIs. In addition, when stratifying for the severity of ABI (GCS ≤ 9 vs > 9), the U-shaped relationship between PaCO2 and in-hospital mortality was consistent. This suggests that both hypocapnia and hypercapnia are associated with increased in-hospital mortality, regardless of GCS status. However, the risk related to forced hypocapnia is more pronounced in patients with GCS ≤ 9, implying that patients with more severe brain injuries are more vulnerable to aggressive reductions in PaCO2.
In TBI patients, and similarly in ICH, PaCO2 between 32 mmHg and 42 mmHg had lower rates of hospital mortality as compared with other acute neurological conditions, suggesting that maintaining these values is associated with better outcomes in this group. In contrast, hypocapnia had a stronger effect on mortality risk in the context of subarachnoid hemorrhage and ischemic stroke, whereas hypercapnia appears less harmful. We can speculate that in TBI and ICH patients, where the main issue pivots on the mass effect related to cerebral edema and bleeding, lower values of PaCO2 can help in managing intracranial hypertension and can mitigate secondary brain damage; on the contrary, in patients with SAH or IS patients who are at risk of vasospasm or cerebral hypoperfusion, hypocapnia can further cause cerebral vasoconstriction and lead to secondary brain damage.
Strengths of our study include the preplanned design, and the use of a large database prospectively collected involving different centers worldwide, which can provide important insights into the state of the art on this topic across different centers. We believe that our results may be of great importance as these provide novelty and deep insights into the optimal values that could potentially be recommended for achieving ventilatory targets [27] in the ABI population with the ultimate goal of improving clinical outcomes. In particular, our results confirm the large use of mild hypocapnia across different centers, and the safety in the use of cutoffs of PaCO2 32–35 mmHg in TBI patients as suggested by Seattle Guidelines [5].
Limitations
This study has several limitations that need to be mentioned. Although our results were obtained from a pre planned secondary analysis, data from the ENIO study are observational and therefore do not allow causality to be inferred from our findings.
In our study, we have used a snapshot approach, with data on PaCO2 available only on days 1, 3, and 7. We acknowledge that this methodology only provides a cross-sectional view of the patients' PaCO2 status at those specific time points and may not reflect the actual time-weighted exposure to different PaCO2 levels. Although continuous monitoring of PaCO2 would be ideal for capturing the duration of exposure, this granularity of data is often not available in multicenter observational studies due to practical constraints; it is also a clinical snapshot of real daily practice, where PaCO2 is typically not monitored continuously.
In addition, the ENIO collected outcome data on in-hospital and ICU mortality, length of stay, and ICU-related complications. No data on Glasgow Outcome Scale extended (GOSE), modified Rankin scale (mRS), and quality of life were available from the main study. Finally, according to the study design, it is not possible to fully understand whether hypo- or hypercapnia was related to an intentional treatment or the patients’ clinical conditions. In this context, a more specific analysis evaluating the association with the outcome of ventilatory variables, such as mode of ventilation, is ongoing and will provide more insights on this topic.
Conclusions
Both hypo- and hypercapnia are associated with in-hospital mortality when considering the whole cohort. However, mild hypocapnia seemed not to be harmful, especially in the TBI subpopulation. Specific targets should be considered according to the type of ABI. Randomized-controlled trials are warranted to confirm our preliminary observational results.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
23 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00134-024-07358-y
References
Hoiland RL, Fisher JA, Ainslie PN (2019) Regulation of the cerebral circulation by arterial carbon dioxide. In: Comprehensive Physiology. Wiley, pp 1101–1154
Ainslie PN, Ashmead JC, Ide K et al (2005) Differential responses to CO 2 and sympathetic stimulation in the cerebral and femoral circulations in humans. J Physiol 566:613–624. https://doi.org/10.1113/jphysiol.2005.087320
Hawryluk GWJ, Aguilera S, Buki A et al (2019) A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med 45:1783–1794. https://doi.org/10.1007/s00134-019-05805-9
Citerio G, Robba C, Rebora P et al (2021) Management of arterial partial pressure of carbon dioxide in the first week after traumatic brain injury: results from the CENTER-TBI study. Intensive Care Med 47:961–973. https://doi.org/10.1007/s00134-021-06470-7
Chesnut R, Aguilera S, Buki A et al (2020) A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med 46:919–929. https://doi.org/10.1007/s00134-019-05900-x
Carney N, Totten AM, O’Reilly C et al (2017) Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 80:6–15
Robba C, Poole D, McNett M et al (2020) Mechanical ventilation in patients with acute brain injury: recommendations of the European Society of Intensive Care Medicine consensus. Intensive Care Med 46:2397–2410. https://doi.org/10.1007/s00134-020-06283-0
Cinotti R, Mijangos JC, Pelosi P, Haenggi M, Gurjar M et al (2022) Extubation in neurocritical care patients: the ENIO international prospective study. Intensive Care Med 48(11):1539–1550. https://doi.org/10.1007/s00134-022-06825-8
von Elm E, Altman DG, Egger M et al (2008) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008
Cinotti R, Pelosi P, Schultz MJ et al (2020) Extubation strategies in neuro-intensive care unit patients and associations with outcomes: the ENIO multicentre international observational study. Ann Transl Med 8:503–503. https://doi.org/10.21037/atm.2020.03.160
Gouvea Bogossian E, Peluso L, Creteur J, Taccone FS (2021) Hyperventilation in adult TBI patients: how to approach it? Front Neurol. https://doi.org/10.3389/fneur.2020.580859
Cleveland WS (1979) Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 74:829–836
Royston P, Sauerbrei W (2008) Multivariable model—building: a pragmatic approach to regression analysis based on fractional polynomials for modelling continuous variables
Feise RJ (2002) Do multiple outcome measures require p value adjustment? J Clin Epidemiol 55:786–789
Jann B (2021) Relative distribution analysis in Stata. Stata J Promot Commun Stat Stata 21:885–951. https://doi.org/10.1177/1536867X211063147
Godoy DA, Lubillo S, Rabinstein AA (2018) Pathophysiology and management of intracranial hypertension and tissular brain hypoxia after severe traumatic brain injury. Neurosurg Clin N Am 29:195–212. https://doi.org/10.1016/j.nec.2017.12.001
Newell DW, Weber JP, Watson R et al (1996) Effect of transient moderate hyperventilation on dynamic cerebral autoregulation after severe head injury. Neurosurgery 39:35–44. https://doi.org/10.1097/00006123-199607000-00008
Steiner LA, Balestreri M, Johnston AJ et al (2005) Effects of moderate hyperventilation on cerebrovascular pressure-reactivity after head injury. Acta Neurochir Suppl 39:35–43. https://doi.org/10.1007/3-211-32318-X_4
Haubrich C, Steiner L, Kim DJ et al (2012) How does moderate hypocapnia affect cerebral autoregulation in response to changes in perfusion pressure in TBI patients? Acta Neurochir Suppl 114:153–156. https://doi.org/10.1007/978-3-7091-0956-4_28
Coles JP, Minhas PS, Fryer TD et al (2002) Effect of hyperventilation on cerebral blood flow in traumatic head injury: clinical relevance and monitoring correlates*. Crit Care Med 30:1950–1959. https://doi.org/10.1097/00003246-200209000-00002
Coles JP, Fryer TD, Coleman MR et al (2007) Hyperventilation following head injury: effect on ischemic burden and cerebral oxidative metabolism*. Crit Care Med 35:568–578. https://doi.org/10.1097/01.CCM.0000254066.37187.88
Marion DW, Puccio A, Wisniewski SR et al (2002) Effect of hyperventilation on extracellular concentrations of glutamate, lactate, pyruvate, and local cerebral blood flow in patients with severe traumatic brain injury*. Crit Care Med 30:2619–2625. https://doi.org/10.1097/00003246-200212000-00001
Brandi G, Stocchetti N, Pagnamenta A et al (2019) Cerebral metabolism is not affected by moderate hyperventilation in patients with traumatic brain injury. Crit Care 23:45. https://doi.org/10.1186/s13054-018-2304-6
Diringer MN, Videen TO, Yundt K et al (2002) Regional cerebrovascular and metabolic effects of hyperventilation after severe traumatic brain injury. J Neurosurg 96:103–108. https://doi.org/10.3171/jns.2002.96.1.0103
Muizelaar JP, Marmarou A, Ward JD et al (1991) Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg 75:731–739. https://doi.org/10.3171/jns.1991.75.5.0731
Bossers SM, Mansvelder F, Loer SA et al (2023) Association between prehospital end-tidal carbon dioxide levels and mortality in patients with suspected severe traumatic brain injury. Intensive Care Med 49:491–504. https://doi.org/10.1007/s00134-023-07012-z
Robba C, Taccone FS, Citerio G (2022) Monitoring cerebral oxygenation in acute brain-injured patients. Intensive Care Med 48:1463–1466. https://doi.org/10.1007/s00134-022-06788-w
Acknowledgements
The authors would like to thank Paolo Pelosi for inspiring the work for this manuscript. ENIO collaborators members: Paër-sélim Abback: Department of Anesthesiology and Critical Care, Beaujon Hospital, DMU Parabol, AP-HP.Nord, 100 Boulevard du General Leclerc, Paris, France.; Anaïs Codorniu: Department of Anesthesiology and Critical Care, Beaujon Hospital, DMU Parabol, AP-HP.Nord, 100 Boulevard du General Leclerc, Paris, France.; Giuseppe Citerio: Neurointensive Care Unit, Ospedale San Gerardo, Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy; Vittoria Ludovica Sala: Neurointensive Care Unit, Ospedale San Gerardo, Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy; Eleonora Tringali: Anesthesia and Intensive Care Unit, A.O.U. Policlinico “G. Rodolico—S. Marco”, Via Santa Sofia 78, Catania; Daniela Alampi : Sapienza Rome University, A.O.U. Sant'Andrea, Via di grottarossa,1035/1039, Rome; Monica Rocco: Sapienza Rome University, A.O.U. Sant'Andrea, Via di grottarossa,1035/1039, Rome; Jessica Giuseppina Maugeri : Arnas Garibaldi Catania, Piazza S. Maria di Gesu' 5, Catania; Agrippino Bellissima : Arnas Garibaldi Catania, Piazza S.Maria Di Gesu' 5, Catania; Matteo Filippini: University Division of Anesthesiology and Critical Care Medicine, ASST Spedali Civili, Piazzale Spedali Civili, 1—Brescia, Brescia, Italy; Nicoletta Lazzeri: University Division of Anesthesiology and Critical Care Medicine, ASST Spedali Civili, Piazzale Spedali Civili, 1—Brescia, Italy; Andrea Cortegiani: Policlinico Paolo Giaccone, Università degli Studi di Palermo, Via del vespro 129, Palermo; Mariachiara Ippolito: Policlinico Paolo Giaccone, Università degli Studi di Palermo, Via del vespro 129, Palermo; Chiara Robba: San Martino Policlinico Hospital—IRCCS for Oncology and Neurosciences—10 Largo Rosanna Benzi; Denise Battaglini: San Martino Policlinico Hospital—IRCCS for Oncology and Neurosciences, 10 Largo Rosanna Benzi; Patrick Biston : CHU Charleroi- Hôpital Civil Marie-Curie, 140 Chaussée de Bruxelles; Mohamed Fathi Al-Gharyani: Benghazi Medical Center, Eastern Selmain-Benghazi-Libya; Russell Chabanne: Clermont-Ferrand University Hospital, Neurocritical Care Unit, Perioperative Medicine Department, 58 rue Montalembert; Léo Astier: Clermont-Ferrand University Hospital, Neurocritical Care Unit, Perioperative Medicine Department, 58 rue Montalembert; Benjamin Soyer: AP-HP, Hôpital Lariboisière, Department of Anesthesia and Critical Care, DMU Parabol, 2 rue Ambroise Paré, Paris, France; Samuel Gaugain: AP-HP, Hôpital Lariboisière, Department of Anesthesia and Critical Care, DMU Parabol, 2 rue Ambroise Paré, Paris, France; Alice Zimmerli: Department of Intensive Care Medicine, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse, Bern; Urs Pietsch: Department of Anaesthesiology and Intensive Care Medicine, Cantonal Hospital St Gallen, Rorschacher Strasse 95; Miodrag Filipovic: Department of Anaesthesiology and Intensive Care Medicine, Cantonal Hospital St Gallen, Rorschacher Strasse 95; Giovanna Brandi: Institute for Intensive Care Medicine, University Hospital of Zurich, Rämistrasse 100; Giulio Bicciato: Institute for Intensive Care Medicine, University Hospital of Zurich, Rämistrasse 100; Ainhoa Serrano: Hospital Clinico Universitario Valencia, Avenida Blasco Ibañez, 17; Berta Monleon: Hospital Clinico Universitario Valencia, Avenida Blasco Ibañez, 17; Peter van Vliet: Haaglanden Medical Center, Lijnbaan 32; Benjamin Marcel Gerretsen: Haaglanden Medical Center, Lijnbaan 32; Iris Xochitl Ortiz-Macias: Hospital Civil de Guadalajara "Fray Antonio Alcalde", Hospital No. 278, Col. El Retiro; Jun Oto: Tokushima University Hospital, 2-50-1, Kuramoto-cho,Tokushima-shi, Tokushima; Noriya Enomoto: Tokushima Prefectural Central Hospital, 1-10-3, Kuramoto-cho,Tokushima-shi,Tokushima; Tomomichi Matsuda: Sapporo Higashi Tokushukai Hospital, 3-1, Kita 33-jo Higashi 14-chome, Higashi-ku, Sapporo; Nobutaka Masui: Sapporo Higashi Tokushukai Hospital, 3-1, Kita 33-jo Higashi 14-chome, Higashi-ku, Sapporo; Pierre Garçon: Service de reanimation, 2-4 Cours de la Gondoire; Jonathan Zarka: Service de reanimation, 2-4 Cours de la Gondoire; Wytze J Vermeijden: Department of Intensive Care, Medics Spectrum Twente MST, Noninsulin 1, Enschede, The Netherlands; Alexander Daniel Cornet: Dep of intensive care, Medics Spectrum Twente MST, Noninsulin 1, Enschede, The Netherlands; Sergio Reyes InfraGard: UMAE Hospital de Traumatology y Orthopedic IMSS, Diagonal Defensor’s de la República sequin 6 Poniente; Rafael Cirino Lara Domínguez: UMAE Hospital de Traumatology y Orthopedic IMSS, Diagonal Defensor’s de la República sequin 6 Poniente; Maria Mercedes Bellini : Hospital Maciel, 25 de Mayo 174; Maria Milagros Gomez Haedo: Hospital Maciel, 25 de Mayo 174; Laura Lamot: Hospital Municipal Leonidas Lucero, Bahia Blanca; Jose Orquera: Sanatoria Pasteur, Chalaco 675; Matthieu BIAIS: Pellegrin SAR Tripoded; Delphine GEORGES: Pellegrin SAR Tripoded; Arvind Baronial: Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, Department of Critical Care Medicine, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow; Roberto Carlos Miranda-Ackerman: Hospital San Javier, v Pablo Casals 640, Prados Providencia, Guadalajara, Jalisco; Francisco José Barbosa-Camacho: Hospital San Javier, Av Pablo Casals 640, Prados Providencia, Guadalajara, Jalisco; John Porter: St George's Hospital, Blackshaw Road, London; Miguel Lopez-Morales: St George's Hospital, Blackshaw Road, London; Thomas Geeraerts: Toulouse University Hospital, Place du Dr Beylic, Toulouse; Baptiste Companion: Toulouse University Hospital, Place du Dr Beylic, Toulouse; David Pérez-Torres: Service de Medicina Intensive, Hospital Universitario Río Ortega, Calle Delaina, 2; Estefanía Prol-Silva: Service de Medicina Intensive, Hospital Universitario Río Ortega, Calle Delaina, 2; Hana Basheer Yahya : Zlitan medical Centre, Kham/Zlitan; Ala Khaled: Abo Selim Trauma Hospital; Mohamed Ghulam: Abo Selim Trauma Hospital; Cracchiolo Neville Andrea: Terapia Intensiva Con Trauma Center Arnas Ospedale Civico Palermo, Piazza n Leotta 4; Palma Maria Daniela: Terapia Intensiva Con Trauma Center Arans Ospedale Civico Palermo, Piazza n Leotta 4; Cristian Deana: Academic Hospital of Udine, Piazzale S.Maria Della Misericordia, 15, Udine; Luigi Vetrugno: University of Chieti-Pescara; Manuel J. Rivera Chavez: Hospital de Alta Especial dad del Baji, Blvd.Milenio #130 Col. San Carlos la Ronchi, León Guanajuato.; Rocio Mendoza Trujillo: Hospital de Alta Especial dad del Baji, Blvd.Milenio #130 Col. San Carlos la Ronchi, León Guanajuato.; Vincent Legros: Department of Anesthesiology and Critical Care, University Hospital of Reims, Hopital Maison Blanche, 45 Rue Cognacq Jay; Benjamin Brochet: Department of Anesthesiology and Critical Care, University Hospital of Reims, Hopital Maison Blanche, 45 Rue Cognacq Jay; Olivier Huet: Department of Anesthesiology and Critical Care, La Cavale Blanche, Boulevard Tanguy Prigent; Marie Geslain: Department of Anesthesiology and Critical Care, La Cavale Blanche, Boulevard Tanguy Prigent; Mathieu van der Jagt: Erasmus MC Rotterdam, dr Molewaterplein 40; Job van Steenkiste: Erasmus MC Rotterdam, dr Molewaterplein 40; Hazem Ahmed: Seoul Clinic; Alexander Edward Coombs: University Hospital Plymouth, Department of Intensive Care Medicine, University Hospital Plymouth, Derriford Road, Plymouth, Devon; Jessie Welbourne: University Hospital Plymouth, Department of Intensive Care Medicine, University Hospital Plymouth, Derriford Road, Plymouth, Devon; Ana Alicia Velarde Pineda: Hospital General Regional # 180 IMSS, Carretera a San Sebastian # 1000 Col. Las cumbres 2 Tlajomulco de Zúñiga; Víctor Hugo Nubert Castillo : Hospital General Regional # 180 IMSS, Carretera a San Sebastian # 1000 Col. Las cumbres 2 Tlajomulco de Zúñiga; Mohammed A. Azab: Cairo University, Giza, Egypt; Ahmed Y. Azzam: Cairo University, Giza, Egypt; David Michael Paul van Meenen: Amsterdam UMC, Meibergdreef 9; Gilberto Adrian Gasca: Hospital Regional de Alta Especial dad de Ixtapaluca, Carretera Federal Mexico -Puebla Km. 34.5, Pueblo de Zoquiapan; Alfredo Arellano: Hospital Regional de Alta Especial dad de Ixtapaluca, Carretera Federal Mexico -Puebla Km. 34.5, Pueblo de Zoquiapan; Forttino Galicia-Espinosa: UMAE Hospital de Traumatología y Orthopedic No 21, IMSS Monterrey, Av. J.M. Pino Suárez S/N esq. 15 de Mayo, Monterrey; José Carlos García-Ramos: UMAE Hospital de Traumatología y Orthopedic No 21, IMSS Monterrey, Av. J.M. Pino Suárez S/N esq. 15 de Mayo, Monterrey; Ghanshyam Yadav: Trauma ICU, Department of Anesthesia, IMS, BHU, Department of Anesthesia, IMS, BHU, Varanasi, India; Amarendra Kumar Jha: Trauma ICU, Department of Anesthesia, IMS, BHU, Department of Anesthesia, IMS, BHU, Varanasi, India; Vincent Robert-Edan: Nantes-Laennec, Hopital Laennec CHU Nantes 1 Bd Jacques Monod; Pierre-Andre Rodie-Talbere: Nantes-Laennec, Hopital Laennec CHU Nantes 1 Bd Jacques Monod; Gaurav Jain: Critical Care Unit, Dept. Of Anaesthesiology and Critical Care, All India Institute of Medical Sciences Rishikesh, All India Institute of Medical Sciences, Virbhadra Marg, Rishikesh; Sagarika Panda: Critical Care Unit, Dept. Of Anaesthesiology and Critical Care, All India Institute of Medical Sciences Rishikesh, All India Institute of Medical Sciences, Virbhadra Marg, Rishikesh; Sonika Agarwal: HIMS, B18/8, HIMS Campus, Dehradun; Yashbir Deewan: HIMS, B18/8, HIMS Campus, Dehradun; Gilberto Adrian Gasca: Hospital Regional de Alta Especial dad de Ixtapaluca, Carretera Federal Mexico -Puebla Km. 34.5, Pueblo de Zoquiapan; Alfredo Arellano: Hospital Regional de Alta Especial dad de Ixtapaluca, Carretera Federal Mexico -Puebla Km. 34.5, Pueblo de Zoquiapan; Syed Tariq Reza: Dhaka Medical College Hospital, Department of Anaesthesia, Analgesia, Palliative & Intensive Care, Dhaka Medical College Hospital, Dhaka, Bangladesh; Md. Mozaffer Hossain: Dhaka Medical College Hospital, Department of Anaesthesia, Analgesia, Palliative & Intensive Care, Dhaka Medical College Hospital, Dhaka, Bangladesh; Christos Papadas: ICU of Asklepieio G.H.A, V. Paulou 1; Vasiliki Chantziara: SAINT SAVVAS hospital, 151 Alexandras Avenue; Chrysanthi Sklavou: SAINT SAVVAS hospital, 151 Alexandras Avenue; Yannick Hourmant: Department of Anesthesiology and Critical Care, Hôtel-Dieu, 1 place Alexis Ricordeau, Nantes, France; Nicolas Grillot: Department of Anesthesiology and Critical Care, Hôtel-Dieu, 1 place Alexis Ricordeau, Nantes, France; Job van Steenkiste: Erasmus Medical Centre, Doctor Molewaterplein 40; Mathieu van der Jagt: Erasmus Medical Centre, Doctor Molewaterplein 40; Romain Pirracchio: Department of Anesthesia and Perioperative Care, University of California, UCSF, 1001 Potrero Ave, San Francisco; Abdelraouf Akkari: Qatar-1, HMC-Doha-Qatar; Mohamed Abdelaty: Qatar-2, HMC-Doha-Qatar; Ahmed Hashim: Qatar-2¸ HMC-Doha-Qatar; Yoann Launey: Department of Anesthesiology and Critical Care, Hopital Pontchaillou, 2 rue Henri le Guilloux, France; Elodie Masseret: Department of Anesthesiology and Critical Care, Hopital Pontchaillou, 2 rue Henri le Guilloux, France; Sigismond Lasocki: Department of Anesthesiology and Critical Care, 4 rue Larrey, Angers, France; Soizic Gergaud: Department of Anesthesiology and Critical Care, 4 rue Larrey, Angers, France; Nicolas Mouclier: Department of Anesthesiology and Critical Care, Hôtel-Dieu, 1 place Alexis Ricordeau, Nantes, France; Sulekha Saxena: Department of Critical Care Medicine, King George's Medical University, Lucknow; Avinash Agrawal: Department of Critical Care Medicine, King George's Medical University, Lucknow; Shakti Bedanta Mishra: IMS and SUM Hospital; Samir Samal: IMS and SUM Hospital; Julio Cesar Mijangos: Hospital Civil de Guadalajara "Fray Antonio Alcalde" Hospital No. 278; Mattias Haënggi: Inselspital, Bern University Hospital; Mohan Gurjar; Marcus J Schultz; Callum Kaye: Aberdeen Royal Infirmary; Daniela Agustin Godoy: Sanatoria Pasteur; Pablo Alvarez: Hospital Maciel; Aikaterini Ioakeimidou: ASKLEPIEIO G.H.A; Yoshitoyo Ueno: Tokushima University Hospital; Rafael Badenes: Hospital Clínico Universitario Valencia; Abdurrahmaan Ali Suei Elbuzidi : Qatar-1; Michaël Piagnerelli : Hôpital Civil Marie-Curie, Université libre de Bruxelles; Muhammed Elhadi : Faculty of Medicine, University of Tripoli, Furnaj; Syed Tariq Reza: Dhaka Medical College Hospital; Mohammed Atef Azab: Cairo University, Giza; Jean Catherine Digitale: University of California, UCSF, 550 16th St; Nicholas Fong: University of California, UCSF 1001 Potrero Ave; Ricardo Campos Cerda: Critical Care Unit, Hospital General Regional no. 46, Instituto Mexicano del Seguro Social, 2063 Lazaro Cárdenas Av; Norma de la Torre Peredo: Critical Care Unit, Hospital General Regional no. 46, Instituto Mexicano del Seguro Social, 2063 Lazaro Cárdenas Av; Victoria McCredie: Toronto Western Hospital-University Health Network, 399 Bathurst St, Toronto, ON M5T 2S8; Elbuzidi Abdurrahmaan Ali Suei: Hamad Medical Corporation, Qatar-1; Matthias Haenggi: Department of Intensive Care Medicine, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse, 3010; Jaber Samir: Montpellier Centre Hospitalier Universitaire de Montpellier, Montpellier (CHU).
Funding
Open access funding provided by Università degli Studi di Genova within the CRUI-CARE Agreement. The work was supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), under Project No. MNESYS (PE0000006)—a multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
Author information
Authors and Affiliations
Consortia
Contributions
CR and RB conceived and designed the manuscript. CR and RB interpreted the data and drafted the manuscript. RB, MS, FT, GC, and CR supervised the work. All authors were involved in critical revision of the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval and consent to participate
Approval to conduct this sub-analysis was not necessary. Approval to enroll patients in the ENIO main study was obtained from the International Review Board (IRB) of the promoter center (Groupe Nantais d’Éthique dans le Domaine de la Santé, IRB No. 7/11/2017), and from the local IRB for each participating center.
Consent for publication
Informed consent was generally waived in accordance with the observational nature of the ENIO study, but if necessary was collected in accordance with the local regulations of each IRB involved. Informed consent was obtained from the patient or from the patient’s neck of kin if the patient was unable to give the consent at the time of enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Names of the ENIO collaborators are listed in Acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Robba, C., Battaglini, D., Abbas, A. et al. Clinical practice and effect of carbon dioxide on outcomes in mechanically ventilated acute brain-injured patients: a secondary analysis of the ENIO study. Intensive Care Med 50, 234–246 (2024). https://doi.org/10.1007/s00134-023-07305-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-023-07305-3