Abstract
Purpose
Increased systemic cortisol availability during adult critical illness is determined by reduced binding-proteins and suppressed breakdown rather than elevated ACTH. Dynamics, drivers and prognostic value of hypercortisolism during pediatric critical illness remain scarcely investigated.
Methods
This preplanned secondary analysis of the PEPaNIC-RCT (N = 1440), after excluding 420 children treated with corticosteroids before PICU-admission, documented (a) plasma ACTH, (free)cortisol and cortisol-metabolism at PICU-admission, day-3 and last PICU-day, their prognostic value, and impact of withholding early parenteral nutrition (PN), (b) the association between corticosteroid-treatment and these hormones, and (c) the association between corticosteroid-treatment and outcome.
Results
ACTH was normal upon PICU-admission and low thereafter (p ≤ 0.0004). Total and free cortisol were only elevated upon PICU-admission (p ≤ 0.0003) and thereafter became normal despite low binding-proteins (p < 0.0001) and persistently suppressed cortisol-metabolism (p ≤ 0.03). Withholding early-PN did not affect this phenotype. On PICU-day-3, high free cortisol and low ACTH independently predicted worse outcome (p ≤ 0.003). Also, corticosteroid-treatment initiated in PICU, which further suppressed ACTH (p < 0.0001), was independently associated with poor outcomes (earlier live PICU-discharge: p < 0.0001, 90-day mortality: p = 0.02).
Conclusion
In critically ill children, systemic cortisol availability is elevated only transiently, much lower than in adults, and not driven by elevated ACTH. Further ACTH lowering by corticosteroid-treatment indicates active feedback inhibition at pituitary level. Beyond PICU-admission-day, low ACTH and high cortisol, and corticosteroid-treatment, predicted poor outcome. This suggests that exogenously increasing cortisol availability during acute critical illness in children may be inappropriate. Future studies on corticosteroid-treatment in critically ill children should plan safety analyses, as harm may be possible.
Similar content being viewed by others
Despite low plasma cortisol binding proteins and reduced cortisol metabolism, systemic cortisol availability in critically ill children is elevated only transiently and to a much lower extent than in adults, and is not driven by elevated ACTH. Beyond the day of PICU-admission, low ACTH and high cortisol, as well as corticosteroid-treatment, independently predicted poor outcome, suggesting that exogenously increasing systemic cortisol availability during acute illness in children may be inappropriate. |
Introduction
Increased systemic cortisol availability is essential to cope with the severe physical stress imposed by surgery, trauma or severe illnesses. It has long been assumed that increased systemic cortisol availability in response to critical illnesses requiring admission to an intensive care unit (ICU) is predominantly driven by a sustained activation of the hypothalamus-pituitary-adrenocortical (HPA) axis, with a 6–10-fold increase in adrenocortical cortisol synthesis and secretion in response to increased ACTH release from the anterior pituitary. Also fasting, often a consequence of severe illness, is considered a metabolic stressor that can affect the HPA axis [1]. In critically ill adults, high as well as low plasma cortisol concentrations have been associated with adverse outcome [2]. More recently, it has become clear that in most adult patients, throughout the first 4 weeks in the ICU, hypercortisolism is present in the face of low or normal rather than high plasma ACTH concentrations, irrespective of whether or not nutritional goals are targeted early [3,4,5]. Instead of being determined by increased ACTH-driven cortisol production, the increased systemic cortisol availability during critical illness in adults was shown to be determined by reduced circulating levels of cortisol binding-proteins with suppressed binding affinity and by a suppressed cortisol breakdown in liver and kidney, which, together, via feedback inhibition, at least partially explain the low plasma ACTH [2,3,4, 6,7,8,9].
Although HPA axis alterations have been described in children suffering from meningococcal sepsis [10,11,12], the dynamics, drivers and prognostic value of systemic cortisol availability in the broader population of critically ill children have only scarcely been investigated [13, 14]. Nevertheless, studies have suggested that a substantial proportion of critically ill children may not be able to increase systemic cortisol availability enough to face the stress of the critical illness, which could cause hemodynamic instability and systemic hyperinflammation with need for vasopressor support [15, 16]. As a consequence, pediatric intensivists often prescribe systemic corticosteroids, often in quite high doses, although adequately powered RCTs investigating its clinical efficacy and safety in children are lacking [15, 16]. It currently remains debated whether or not such treatment improves morbidity and mortality of critically ill children and some studies have suggested possible harm [17,18,19,20].
In this study, we aimed at (a) documenting changes over time during the course of pediatric critical illness in plasma ACTH, (free)cortisol and cortisol metabolites, their prognostic value and the impact of withholding early supplemental parenteral nutrition (PN), (b) investigating the association between treatment with systemic corticosteroids and these parameters, and (c) assessing the independent association between treatment with systemic corticosteroids and patient-centered clinical outcomes.
Materials and methods
Patients and sample collection
This study was a preplanned secondary analysis of the multicenter PEPaNIC randomized controlled trial (RCT) conducted in Belgium, the Netherlands and Canada (June 2012–July 2015) [21]. Written informed consent was obtained from parents or legal guardians. The institutional ethical review board at each participating center approved the protocol and consent forms of the study (ML8052, NL38772.000.12, Pro00038098), which was performed in accordance to the 1964 Declaration of Helsinki and later amendments. The detailed study protocol and primary results have been published [21, 22].
The PEPaNIC trial investigated the clinical outcomes of withholding supplemental parenteral nutrition (PN) up to day 8 in the pediatric ICU (PICU), referred to as “late-PN”, as compared with early supplemental PN whenever enteral nutrition alone was insufficient to reach the caloric target, referred to as “early-PN”. Children aged 0–17 years were eligible for inclusion, if a PICU-stay of 24 h or more was expected, if they had a moderate or severe risk of malnutrition (score ≥ 2 on the Screening Tool for Risk on Nutritional Status and Growth (STRONGkids)), and if none of the predefined exclusion criteria were met.
Enteral nutrition was started as soon as possible in both groups. In the early-PN group, supplemental PN was initiated within 24 h after PICU-admission to reach the caloric target (Online Resource Table_1-2), whereas PN was withheld up to the morning of PICU day 8 for patients in the late-PN group. Although labeled “late-PN” group, the majority of these patients needed intensive care for less than 8 days and thus did not receive PN. In the late-PN group, a mixture of intravenous dextrose (5%) and saline was administered to match the intravenous fluid load given to the early-PN patients. Patients in both groups equally received intravenous trace elements, minerals and vitamins, and blood glucose control with insulin according to local targets. Blood was sampled upon admission and then daily at 6 a.m. until PICU-discharge or death, within limitations for amount of blood sampling allowed by the institutional ethical review boards per center. For the quantification of hormonal parameters of the HPA axis, blood samples were collected in pre-chilled EDTA tubes and immediately placed on ice, centrifuged at 4 °C, with plasma stored at − 80 °C until assay. Daily urine samples from a 24-h urine collection were stored in Vacuette urine tubes at − 80 °C until quantification of the cortisol metabolites.
To investigate the time course of the changes in plasma concentrations of ACTH, total cortisol, free cortisol, CBG and albumin during critical illness in children (Fig. 1, Panel_A.1), patients who received corticosteroids within 3 days before inclusion (including in the emergency room) or prior to the blood sampling day were excluded, as well as those patients who died in PICU on the day of blood sampling to avoid bias evoked by the agonal stress response. Of the remaining patients, we included those 442 patients for whom plasma samples were available upon admission and on day 3 (for 230 long-stay patients treated in PICU for at least 3 days, which is the median duration of PICU stay, Table 1) or last PICU day (for 212 short-stay patients treated in PICU for < 3 days). For comparison, 64 healthy children, who had never been critically ill and from whom blood was drawn immediately after intravenous catheterization prior to minor elective surgery, age- and gender-matched with the patients, were included (Fig. 1, Panel_A.1). The prognostic value for the clinical outcomes (new infection acquired in the PICU, time to live discharge from PICU and 90-day mortality) of the admission plasma concentrations of ACTH and free cortisol was assessed among these 442 patients (Fig. 1, Panel_A.4). The prognostic value of plasma ACTH and free cortisol on day 3 was also assessed for the 230 long-stay patients (Fig. 1, Panel_A.4). For the estimation of the activity of cortisol-metabolizing enzymes via urinary cortisol metabolites, a representative subset of 76 patients and 25 age- and gender-matched healthy control children was selected (Fig. 1, Panel_A.2).
Diagram of the study design. ACTH adrenocorticotropic hormone, CBG cortisol-binding globulin, CS corticosteroids, D3 day 3 in PICU, HPA axis hypothalamus–pituitary–adrenal axis, LD last day in PICU, PEPaNIC early versus late parenteral nutrition in the pediatric intensive care unit, PICU pediatric intensive care unit, PN parenteral nutrition, RCT randomized controlled trial. Short-stay patients are patients who needed intensive care for 1 or 2 days, long-stay patients are patients who needed intensive care for at least 3 days
To investigate whether late-PN, as compared with early-PN, altered the changes in endogenous, illness-induced HPA axis parameters, between PICU admission and day 3 or last PICU day for patients discharged earlier (Fig. 1, Panel_A.3), a propensity score-matched patient subset was selected, given the required exclusion of a substantial number of patients. Propensity scores were estimated with logistic regression based on baseline risk factors as covariates, including treatment center, risk of malnutrition (STRONGkids score), age group, diagnosis upon admission, severity of illness (PeLOD score reflecting degree of organ failure and PIM2 score estimating risk of death) and sepsis upon admission [23,24,25,26]. One-to-one nearest neighbor-matching without replacement and a caliper of 0.3 retained 414 patients.
To investigate the association between treatment with corticosteroids in the PICU and plasma concentrations of ACTH, total cortisol, free cortisol, CBG and albumin (Fig. 1, Panel_2), the 33 patients who had a measurement the morning after the first day of corticosteroid-treatment were matched case-by-case to patients who had not been treated with corticosteroids and for whom a measurement was available for the same PICU-stay day (Fig. 1, Panel_2, Online Resource Methods_1). Distinction was made between hydrocortisone and synthetic corticosteroids given that the latter are not detected by the assay used for quantifying cortisol.
To investigate the independent association between treatment with corticosteroids initiated in the PICU and patient-centered clinical outcomes (new infection acquired in the PICU, time to live discharge from PICU and 90-day mortality) (Fig. 1, Panel_4), all 1020 patients who had not been treated with corticosteroids before PICU admission were included.
Plasma and urinary analyses
Plasma ACTH concentrations were quantified by double-monoclonal immunoradiometric assay (Brahms Diagnostics) and plasma cortisol (Immunotech) and cortisol-binding-globulin (CBG) (Riazen) by competitive radioimmunoassay. Plasma albumin was quantified by the bromocresol-green colorimetric method (Sigma-Aldrich). Plasma free cortisol was calculated with the Coolens formula adapted for individual albumin and CBG concentrations, which has previously been validated as representative for measured plasma free cortisol concentrations in the ICU context [6, 27]. After deglucoronidation, the absolute urinary concentrations of cortisol (F), 5α-tetrahydrocortisol (allo-THF), 5β-tetrahydrocortisol (THF), cortisone (E), 5α-tetrahydrocortisone (allo-THE) and tetrahydrocortisone (THE) were determined with liquid chromatography-tandem mass spectrometry (LC–MS/MS) as reported previously [4].
Statistical analyses
For research question A and C (Fig. 1), sample size was determined by availability. For research question B (Fig. 1), given lack of data for children in the PICU, sample size was determined based on published data regarding the impact of exogenous corticosteroids on plasma ACTH outside the context of critical illness [28, 29]. To detect a lowering in plasma ACTH in this order of magnitude, with 80% power and 95% certainty, 33 patients per group were required.
Continuous data and proportions were compared with Wilcoxon Rank Sum and Chi square tests, respectively. Data are presented as medians and interquartile ranges or numbers and percentages.
Independent associations of plasma concentrations of ACTH and free cortisol, or of corticosteroid-treatment, with patient-centered clinical outcomes were analyzed with multivariable logistic regression and proportional hazard analyses, adjusted for baseline risk factors [treatment center, risk of malnutrition (STRONGkids score), age group, diagnosis upon admission, severity of illness (PeLOD and PIM2), sepsis upon admission] [23,24,25,26]. Effect sizes are reported as odds ratios (OR) and hazard ratios (HR), respectively, with 95% confidence intervals (CI).
Propensity score matching was performed with SPSS R-menu R3.1 (Foundation for Statistical Computing) in IBM SPSS Statistics 25.0.0.0 (SPSS, Chicago, IL). All analyses were performed with JMP®Pro14.0.0 (SAS Institute Inc, Cary, NC). Two-sided p values < 0.05 were considered to indicate statistical significance.
Results
For the three main research questions, depicted in Fig. 1, comparisons of the patient characteristics are provided in Table 1 and Online Resource Table_3-4.
Time course of the changes in plasma concentrations of ACTH, total cortisol, free cortisol, CBG, and albumin and estimations of cortisol metabolism during critical illness, prognostic value and impact of late-PN versus early-PN
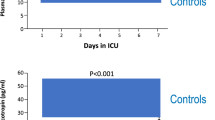
Upon PICU-admission, plasma ACTH concentrations were not significantly different from those in the matched healthy children and became low thereafter throughout PICU stay (Fig. 2a). Plasma total cortisol concentrations were only above normal at PICU-admission and became normal thereafter (Fig. 2b). Also, plasma free cortisol concentrations were only higher than in healthy children upon PICU-admission. Thereafter, plasma free cortisol levels were no longer elevated (Fig. 2c), despite plasma CBG and albumin concentrations that were mostly low (Fig. 2d, e) and despite the observation that the estimated activities of cortisol-metabolizing enzymes, 11β-hydroxysteroid-dehydrogenase-2 and the A-ring reductases 5α-reductase and 5β-reductase, remained low throughout PICU stay (Fig. 2f–i).
Time course of the changes in plasma concentrations of ACTH, total cortisol, free cortisol, CBG and albumin, and estimations of cortisol metabolism during critical illness. Plasma concentrations of ACTH (a), total cortisol (b), free cortisol (c), CBG (d) and albumin (e) were quantified, and activities of the cortisol metabolizing enzymes 11β-HSD2 (f), 5α-reductase (g) and 5β-reductase (h, i) were estimated upon PICU admission, on PICU day 3 or last PICU day for patients with a shorter PICU stay, and on the last PICU day beyond day 3 if still in PICU beyond day 3. Results overall apply irrespective of the presence of sepsis, and irrespective of whether patients were admitted after cardiac surgery or for other reasons. Box plots show medians, interquartile ranges, and 10th and 90th percentiles. The grey bars represent the interquartile ranges obtained for the healthy control children, with the white lines indicating the medians. ACTH: adrenocorticotropic hormone, aTHF: allo-THF (5α-tetrahydrocortisol), 11β-HSD2: 11β-hydroxysteroid-dehydrogenase type 2, CBG: cortisol-binding globulin, D3: day 3 in PICU, E: cortisone, F: cortisol, LD short-stay: last day in PICU for patients who needed intensive care for 1 or 2 days, LD long-stay: last day in PICU beyond day 3 for long-stay patients who needed intensive care for at least 3 days, PICU: pediatric intensive care unit, THE: tetrahydrocortisone, THF: 5β-tetrahydrocortisol
The use of late-PN, as compared with early-PN, did not affect the changes over time in plasma concentrations of ACTH, (free)cortisol, CBG, or albumin (Online Resource Fig. 1).
Adjusted for baseline risk factors, including type and severity of illness among others, upon PICU-admission plasma concentrations of ACTH and free cortisol were not independently associated with the clinical outcomes (Online Resource Table_5). However, among long-stay patients, higher plasma ACTH concentrations on day 3 in the PICU were independently indicative of a higher likelihood of earlier live discharge from PICU, whereas higher plasma free cortisol concentrations on that day were independently indicative of a lower likelihood of earlier live discharge from PICU (Table 2). There were no independent associations between plasma ACTH or free cortisol on day 3 and the risk of new infections or the risk of 90-day mortality.
Association between treatment with corticosteroids initiated in the PICU and plasma concentrations of ACTH, total cortisol, free cortisol, CBG and albumin
Among the 33 selected patients who received corticosteroids on median day 2 (IQR 1-3), 28 received synthetic corticosteroids and 5 received hydrocortisone. Corticosteroid-treatment further suppressed plasma concentrations of ACTH as compared with the 33 matched patients who did not receive corticosteroids and were also assessed on median day 2 (IQR 1-3) (Fig. 3). Expectedly, the use of synthetic corticosteroids lowered plasma total and free cortisol concentrations (Fig. 3), whereas hydrocortisone increased plasma total and free cortisol. There was no association with the plasma concentrations of CBG or albumin.
Association between treatment with corticosteroids initiated in the PICU and plasma concentrations of ACTH, total cortisol, free cortisol, CBG, and albumin. Plasma concentrations of ACTH (a), total cortisol (b), free cortisol (c), CBG (d) and albumin (e) were quantified on the morning after the first day of CS administration and on equivalent days for patients who did not receive corticosteroids, manually matched 1:1 with the patients who received CS. To evaluate the association between CS administration and total and free cortisol, a distinction was made between hydrocortisone and synthetic CS, since the radioimmunoassay for cortisol does not measure synthetic CS. Box plots show medians, interquartile ranges, and 10th and 90th percentiles. The grey bars represent the interquartile range obtained for the healthy control children, with the white lines indicating the medians. P values represent the comparison of CS (or type of CS) versus no CS administration. ACTH: adrenocorticotropic hormone, CBG: cortisol-binding globulin, CS: corticosteroids, PICU: pediatric intensive care unit, PN: parenteral nutrition
Independent association between treatment with corticosteroids initiated in the PICU and patient-centered clinical outcomes
Of the 1020 patients who had not been treated with corticosteroids prior to PICU admission, 270 (26.5%) received corticosteroids initiated on median day 2 (IQR 1–5) after admission to the PICU. Among the 652 long-stay patients in PICU for at least 3 days, 217 (33.3%) received corticosteroids initiated on median day 3 (IQR 1–6) after PICU admission. Adjusted for baseline risk factors, the use of corticosteroids during PICU stay was independently associated with a lower likelihood of an earlier live discharge from ICU and with a higher risk of 90-day mortality for all patients (Online Resource Table_6) and for long-stay patients alike (Table 2). There was no independent association between use of corticosteroids and the risk of new infections.
Discussion
In this study of critically ill children admitted to the PICU, plasma ACTH concentrations were either normal or low and were further lowered by the use of exogenous corticosteroids. Total and free plasma cortisol levels were elevated only briefly, thereafter lowering to normal levels despite persistently low circulating levels of cortisol binding-proteins and despite suppressed cortisol metabolism throughout PICU stay. Withholding supplemental PN during the first week in PICU did not affect this phenotype. Adjusted for risk factors, upon admission plasma concentrations of ACTH or free cortisol did not hold prognostic value for patient-centered clinical outcomes whereas on day 3 in the PICU, a high free cortisol and a low plasma ACTH level independently predicted worse outcome. Also, treatment with systemic corticosteroids initiated during the first few days in the PICU, which further suppressed ACTH, was found to independently associate with poor outcomes, adjusted for other risk factors.
Systemic cortisol availability in critically ill children treated in the PICU was found to be elevated only transiently and to a much lesser extent than was previously reported for adults [3, 5, 30]. The finding that plasma ACTH in patients was never significantly higher than in matched healthy children, not even in the presence of elevated plasma cortisol upon PICU-admission, was in line with previous adult data [3, 5, 30]. These findings suggest that peripheral mechanisms, such as low plasma binding and suppressed cortisol breakdown, are active to increase and maintain cortisol availability also in the pediatric critically ill population [3, 9]. The observation that exogenous corticosteroids further suppressed plasma ACTH suggests that ACTH is not fully endogenously suppressed during pediatric critical illness and thus that central activators balance against suppressors in exogenous corticosteroid-naive patients but that pituitary feedback inhibition remains responsive to a further increase of glucocorticoid signaling.
The low plasma ACTH observed beyond the PICU-admission day in the critically ill children cannot be brought about by feedback-inhibition through free cortisol, given that free cortisol was no longer elevated, unless the set-point for feedback inhibition would be lower in this context. A lower set-point for feedback inhibition by thyroid hormones has also been suggested as an explanation for the low T3 syndrome of critical illness [31,32,33]. Absence of elevated free cortisol concentrations after a few days in the PICU, while cortisol binding-proteins were low and cortisol metabolism was suppressed, suggests that the concomitantly low ACTH must have played a role. Endogenous or iatrogenic suppressors of ACTH may be involved. Candidate endogenous suppressors of ACTH are elevated levels of bile acids as these have shown to be able to cross the blood–brain barrier and act as ligands for the glucocorticoid receptor, hereby potentially suppressing corticotropin-releasing hormone and hereby suppressing ACTH [34,35,36]. The use of opioids and other drugs in the PICU may have added suppressive effects on the HPA axis [30, 37, 38].
Admission levels of plasma ACTH and cortisol did not hold prognostic value for clinical outcome when adjusted for type and severity of illness and other risk factors. Hence, they appeared to be merely a correlate of the type and severity of illness. However, it was striking to observe that among long-stay patients, after extensive adjustment for risk factors including type and severity of illness, a high plasma free cortisol and a low plasma ACTH on day 3, a time point where both cortisol and ACTH levels were lowered as compared with PICU admission, together were independently predictive of poor outcome. Also, exogenous steroid treatment initiated during the first few days in the PICU, which further lowered plasma ACTH, was predictive of poor outcome after extensive adjustment for risk factors. Together these findings may suggest that the amount of cortisol availability required for an effective struggle for survival and recovery from critical illness in children may not be as high as previously assumed and lower than in adults. Excessive amounts of cortisol could indeed also be harmful [18, 19]. Alternatively, a direct role of endogenous and/or exogenous ACTH suppressors in mediating the independent association between low plasma ACTH and adverse outcome is possible and cannot be excluded from this study.
The strengths of this study are the large sample size of a heterogeneous cohort of critically ill children and the use of samples and data from a well-documented RCT which allowed to carefully adjust for prospectively registered confounders and which allowed case-by-case matching. A weakness of the study is that data were predominantly gathered during the first week in the PICU so that no conclusions can be drawn on a more prolonged phase of pediatric critical illness. Another weakness is the observational nature of our analyses of the associations with patient-centered clinical outcomes, which (although adjusted for baseline risk factors) cannot fully take into account potential confounding factors such as disease course. Lastly, free cortisol concentrations were not directly measured but calculated.
In conclusion, cortisol availability during pediatric critical illness is only briefly elevated, driven by peripheral rather than central mechanisms, and is followed by low ACTH and normal free cortisol levels until PICU-discharge. Low ACTH is further lowered by treatment with corticosteroids suggesting active feedback inhibition at the pituitary level. Beyond PICU-admission day, a high circulating cortisol as well as the administration of systemic corticosteroids appeared independent predictors of adverse outcome. These findings raise the hypothesis that increasing cortisol availability during the first days after onset of critical illness in children may be inappropriate. Studies investigating the effect of treatment with corticosteroids in critically ill children should thus carefully plan interim safety analyses to avoid possible harm.
References
Rohleder N, Kirschbaum C (2007) Effects of nutrition on neuro-endocrine stress responses. Curr Opin Clin Nutr Metab Care 10(4):504–510. https://doi.org/10.1097/MCO.0b013e3281e38808
Teblick A, Peeters B, Langouche L, Van den Berghe G (2019) Adrenal function and dysfunction in critically ill patients. Nat Rev Endocrinol 15(7):417–427. https://doi.org/10.1038/s41574-019-0185-7
Boonen E, Vervenne H, Meersseman P, Andrew R, Mortier L, Declercq PE, Vanwijngaerden YM, Spriet I, Wouters PJ, Vander Perre S, Langouche L, Vanhorebeek I, Walker BR, Van den Berghe G (2013) Reduced cortisol metabolism during critical illness. N Engl J Med 368(16):1477–1488. https://doi.org/10.1056/NEJMoa1214969
Peeters B, Meersseman P, Vander Perre S, Wouters PJ, Vanmarcke D, Debaveye Y, Billen J, Vermeersch P, Langouche L, Van den Berghe G (2018) Adrenocortical function during prolonged critical illness and beyond: a prospective observational study. Intensive Care Med 44(10):1720–1729. https://doi.org/10.1007/s00134-018-5366-7
Meersseman P, Boonen E, Peeters B, Vander Perre S, Wouters PJ, Langouche L, Van den Berghe G (2015) Effect of early parenteral nutrition on the HPA axis and on treatment with corticosteroids in intensive care patients. J Clin Endocrinol Metab 100(7):2613–2620. https://doi.org/10.1210/jc.2015-1846
Boonen E, Meersseman P, Vervenne H, Meyfroidt G, Guiza F, Wouters PJ, Veldhuis JD, Van den Berghe G (2014) Reduced nocturnal ACTH-driven cortisol secretion during critical illness. Am J Physiol Endocrinol Metab 306(8):E883–E892. https://doi.org/10.1152/ajpendo.00009.2014
Pemberton PA, Stein PE, Pepys MB, Potter JM, Carrell RW (1988) Hormone binding globulins undergo serpin conformational change in inflammation. Nature 336(6196):257–258. https://doi.org/10.1038/336257a0
Henley D, Lightman S, Carrell R (2016) Cortisol and CBG—getting cortisol to the right place at the right time. Pharmacol Ther 166:128–135. https://doi.org/10.1016/j.pharmthera.2016.06.020
Hamrahian AH, Oseni TS, Arafah BM (2004) Measurements of serum free cortisol in critically ill patients. N Engl J Med 350(16):1629–1638. https://doi.org/10.1056/NEJMoa020266
De Kleijn ED, Joosten KF, Van Rijn B, Westerterp M, De Groot R, Hokken-Koelega AC, Hazelzet JA (2002) Low serum cortisol in combination with high adrenocorticotrophic hormone concentrations are associated with poor outcome in children with severe meningococcal disease. Pediatr Infect Dis J 21(4):330–336. https://doi.org/10.1097/00006454-200204000-00013
den Brinker M, Hokken-Koelega AC, Hazelzet JA, de Jong FH, Hop WC, Joosten KF (2008) One single dose of etomidate negatively influences adrenocortical performance for at least 24 h in children with meningococcal sepsis. Intensive Care Med 34(1):163–168. https://doi.org/10.1007/s00134-007-0836-3
den Brinker M, Joosten KF, Liem O, de Jong FH, Hop WC, Hazelzet JA, van Dijk M, Hokken-Koelega AC (2005) Adrenal insufficiency in meningococcal sepsis: bioavailable cortisol levels and impact of interleukin-6 levels and intubation with etomidate on adrenal function and mortality. J Clin Endocrinol Metab 90(9):5110–5117. https://doi.org/10.1210/jc.2005-1107
Aydin BK, Demirkol D, Bas F, Turkoglu U, Kumral A, Karabocuoglu M, Citak A, Darendeliler F (2014) Evaluation of endocrine function in children admitted to pediatric intensive care unit. Pediatr Int 56(3):349–353. https://doi.org/10.1111/ped.12269
Poomthavorn P, Lertbunrian R, Preutthipan A, Sriphrapradang A, Khlairit P, Mahachoklertwattana P (2009) Serum free cortisol index, free cortisol, and total cortisol in critically ill children. Intensive Care Med 35(7):1281–1285. https://doi.org/10.1007/s00134-009-1480-x
Menon K, Ward RE, Lawson ML, Gaboury I, Hutchison JS, Hebert PC, Canadian Critical Care Trials Group (2010) A prospective multicenter study of adrenal function in critically ill children. Am J Respir Crit Care Med 182(2):246–251. https://doi.org/10.1164/rccm.200911-1738OC
Dupuis C, Thomas S, Faure P, Gayot A, Desrumaux A, Wroblewski I, Debillon T, Emeriaud G (2010) Secondary adrenal insufficiency in the acute phase of pediatric traumatic brain injury. Intensive Care Med 36(11):1906–1913. https://doi.org/10.1007/s00134-010-2012-4
Nichols B, Kubis S, Hewlett J, Yehya N, Srinivasan V (2017) Hydrocortisone therapy in catecholamine-resistant pediatric septic shock: a pragmatic analysis of clinician practice and association with outcomes. Pediatr Crit Care Med 18(9):e406–e414. https://doi.org/10.1097/PCC.0000000000001237
Menon K, McNally JD, Zimmerman JJ, Agus MS, O’Hearn K, Watson RS, Wong HR, Duffett M, Wypij D, Choong K (2017) Primary outcome measures in pediatric septic shock trials: a systematic review. Pediatr Crit Care Med 18(3):e146–e154. https://doi.org/10.1097/PCC.0000000000001078
Menon K, McNally JD, Choong K, Lawson ML, Ramsay T, Hutchison JS, Foster J, Wong HR, Canadian Critical Care Trials Group SI (2015) A cohort study of pediatric shock: frequency of corticosteriod use and association with clinical outcomes. Shock 44(5):402–409. https://doi.org/10.1097/SHK.0000000000000355
Menon K, Wong HR (2015) Corticosteroids in pediatric shock: a call to arms. Pediatr Crit Care Med 16(8):e313–e317. https://doi.org/10.1097/PCC.0000000000000513
Fivez T, Kerklaan D, Verbruggen S, Vanhorebeek I, Verstraete S, Tibboel D, Guerra GG, Wouters PJ, Joffe A, Joosten K, Mesotten D, Van den Berghe G (2015) Impact of withholding early parenteral nutrition completing enteral nutrition in pediatric critically ill patients (PEPaNIC trial): study protocol for a randomized controlled trial. Trials 16:202. https://doi.org/10.1186/s13063-015-0728-8
Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, Debaveye Y, Vlasselaers D, Desmet L, Casaer MP, Garcia Guerra G, Hanot J, Joffe A, Tibboel D, Joosten K, Van den Berghe G (2016) Early versus late parenteral nutrition in critically Ill children. N Engl J Med 374(12):1111–1122. https://doi.org/10.1056/NEJMoa1514762
Hulst JM, Zwart H, Hop WC, Joosten KF (2010) Dutch national survey to test the STRONGkids nutritional risk screening tool in hospitalized children. Clin Nutr 29(1):106–111. https://doi.org/10.1016/j.clnu.2009.07.006
Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, Lacroix J, Leclerc F (2003) Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet 362(9379):192–197. https://doi.org/10.1016/S0140-6736(03)13908-6
Slater A, Shann F, Pearson G, Paediatric Index of Mortality Study Group (2003) PIM2: a revised version of the paediatric index of mortality. Intensive Care Med 29(2):278–285. https://doi.org/10.1007/s00134-002-1601-2
Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis (2005) International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6(1):2–8. https://doi.org/10.1097/01.pcc.0000149131.72248.e6
Vanhorebeek I, Peeters RP, Vander Perre S, Jans I, Wouters PJ, Skogstrand K, Hansen TK, Bouillon R, Van den Berghe G (2006) Cortisol response to critical illness: effect of intensive insulin therapy. J Clin Endocrinol Metab 91(10):3803–3813. https://doi.org/10.1210/jc.2005-2089
Pasquali R, Ambrosi B, Armanini D, Cavagnini F, Uberti ED, Del Rio G, de Pergola G, Maccario M, Mantero F, Marugo M, Rotella CM, Vettor R, Study Group on Obesity of the Italian Society of European (2002) Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: a dose-response study. J Clin Endocrinol Metab 87(1):166–175. https://doi.org/10.1210/jcem.87.1.8158
Goto M, Miyagawa N, Kikunaga K, Miura M, Hasegawa Y (2015) High incidence of adrenal suppression in children with Kawasaki disease treated with intravenous immunoglobulin plus prednisolone. Endocr J 62(2):145–151. https://doi.org/10.1507/endocrj.EJ14-0385
Peeters B, Guiza F, Boonen E, Meersseman P, Langouche L, Van den Berghe G (2017) Drug-induced HPA axis alterations during acute critical illness: a multivariable association study. Clin Endocrinol (Oxf) 86(1):26–36. https://doi.org/10.1111/cen.13155
Fliers E, Guldenaar SE, Wiersinga WM, Swaab DF (1997) Decreased hypothalamic thyrotropin-releasing hormone gene expression in patients with nonthyroidal illness. J Clin Endocrinol Metab 82(12):4032–4036. https://doi.org/10.1210/jcem.82.12.4404
Boelen A, Kwakkel J, Chassande O, Fliers E (2009) Thyroid hormone receptor beta mediates acute illness-induced alterations in central thyroid hormone metabolism. J Neuroendocrinol 21(5):465–472. https://doi.org/10.1111/j.1365-2826.2009.01863.x
Boelen A, Kwakkel J, Fliers E (2011) Beyond low plasma T3: local thyroid hormone metabolism during inflammation and infection. Endocr Rev 32(5):670–693. https://doi.org/10.1210/er.2011-0007
Vanwijngaerden YM, Wauters J, Langouche L, Vander Perre S, Liddle C, Coulter S, Vanderborght S, Roskams T, Wilmer A, Van den Berghe G, Mesotten D (2011) Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology 54(5):1741–1752. https://doi.org/10.1002/hep.24582
Swain MG, Patchev V, Vergalla J, Chrousos G, Jones EA (1993) Suppression of hypothalamic-pituitary-adrenal axis responsiveness to stress in a rat model of acute cholestasis. J Clin Invest 91(5):1903–1908. https://doi.org/10.1172/JCI116408
McMillin M, Frampton G, Quinn M, Divan A, Grant S, Patel N, Newell-Rogers K, DeMorrow S (2015) Suppression of the HPA axis during cholestasis can be attributed to hypothalamic bile acid signaling. Mol Endocrinol 29(12):1720–1730. https://doi.org/10.1210/me.2015-1087
Vuong C, Van Uum SH, O’Dell LE, Lutfy K, Friedman TC (2010) The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev 31(1):98–132. https://doi.org/10.1210/er.2009-0009
Besnier E, Clavier T, Compere V (2017) The hypothalamic-pituitary-adrenal axis and anesthetics: a review. Anesth Analg 124(4):1181–1189. https://doi.org/10.1213/ANE.0000000000001580
Acknowledgements
We acknowledge the work of the research nurses and nurses and doctors of the PICU, who collected and entered the data. We thank Nele Peersman for her expertise and assistance in the laboratory analyses of the urinary cortisol metabolites. We would also like to thank the healthy children, patients admitted to the PICU and their parents for participating in the study. This study was supported by the Methusalem program of the Flemish government (METH14/06 to IV and GVdB), the Flemish Agency for Innovation through Science and Technology (IWT-TBM110685 to GVdB), the European Research Council (Advanced Research Grants AdvG-2012-321670 and AdvG-2017-785809 to GVdB), Fonds NutsOhra (to SV), Sophia Research Foundation (SSWO, to SV), Stichting Agis Zorginnovatie (to SV), Erasmus Trustfonds (to SV) and the European Society for Clinical Nutrition and Metabolism (ESPEN research grant to SV). The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committees (Medical Ethical Committee University Hospitals Leuven: ML8052, Medical Ethical Review Committee Erasmus Medical Center Rotterdam: NL38772.000.12, Health Research Ethics Board Stollery Children’s Hospital Edmonton: Pro00038098) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jacobs, A., Derese, I., Vander Perre, S. et al. Dynamics and prognostic value of the hypothalamus–pituitary–adrenal axis responses to pediatric critical illness and association with corticosteroid treatment: a prospective observational study. Intensive Care Med 46, 70–81 (2020). https://doi.org/10.1007/s00134-019-05854-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05854-0