Abstract
Purpose
To compare four methods of volume recruitment upon initiation of high frequency oscillatory ventilation (HFOV).
Methods
Anesthetized intubated neonatal piglets (n = 10) underwent repeated saline lavage, followed by conventional mechanical ventilation (CMV). After transition to HFOV at a mean airway pressure 8 cmH2O above CMV (P basal), four methods of lung volume recruitment were tested in each animal in random order: Escalating—step-wise pressure increments over 6 min to a peak mean airway pressure 12 cmH2O above P basal; Sustained dynamic inflation (DI)—a 20 s inflation to the same peak pressure; DI repeated six times for 1 s; Standard—mean airway pressure set directly at P basal. After each recruitment method, HFOV continued at P basal for 15 min. Thoracic gas volume and distribution of aeration were determined by single slice computed tomography, and oxygenation by arterial blood gas sampling.
Results
Escalating recruitment resulted in the greatest thoracic gas volume 15 min post-recruitment [77 ± 3.3% of total lung capacity vs. 70 ± 4.2% (Sustained DI), 65 ± 3.5% (Repeated DI), 63 ± 5.1% (Standard); mean ± SEM; P = 0.042, ANOVA]. All methods resulted in a reduction in non-aerated lung, with the greatest redistribution to normally aerated lung being with Escalating recruitment. Oxygenation 15 min post-recruitment was better with the Escalating method than with Repeated DI or Standard recruitment (pO2 307 ± 41 vs. 159 ± 36 vs. 134 ± 39 mmHg, respectively; P = 0.016, ANOVA).
Conclusions
Escalating recruitment produced the greatest increase in lung volume and resolution of atelectasis, and is recommended for lung volume recruitment upon initiation of HFOV.
Similar content being viewed by others
Introduction
High frequency oscillatory ventilation (HFOV) is a mode of ventilation that is widely used in the treatment of neonatal respiratory failure [1]. Meta-analysis of randomized controlled clinical trials in ventilated preterm infants suggests that when used with a sufficiently high lung volume, HFOV may reduce the incidence of ventilator-induced lung injury and chronic lung disease, without any increase in risk of neurological sequelae [2]. Moreover, laboratory studies [3–5] and recent clinical observations [6] have shown that if the lung is inflated to near total capacity, mean airway pressure (P AW) can then be reduced to allow HFOV to be applied near the point of maximal curvature of the deflation limb of the pressure–volume relationship of the lung, now appreciated to be the optimal region in which to apply tidal ventilation [7, 8].
Whilst the importance of adequate lung volume in applying HFOV is unquestionable, there are few experimental data upon which to base a pressure strategy to achieve good lung inflation as HFOV is commenced. Most clinical trials have stipulated initiation of HFOV at a P AW 2–4 cmH2O higher than on conventional mechanical ventilation (CMV), with further adjustment thereafter only if oxygenation remains compromised [9, 10]. Other investigators have adopted a more active approach to lung recruitment, using a transient 5–10 s increase in P AW with HFOV continuing (dynamic inflation, DI) [11], or stepwise increments in P AW until oxygenation improves [12–14], followed by return to a lower maintenance P AW. Such pressure strategies consistently improve oxygenation during HFOV, and have been seen to result in a higher lung volume in both experimental animals [3–5, 15, 16] and human infants [6]. Whether volume recruitment by these methods results in greater re-expansion of atelectatic lung units during HFOV is not known, nor are there experimental data on the risk of overdistension using such techniques.
In the evaluation of lung volume change and distribution of aeration during mechanical ventilation, computed tomography (CT) has become an important investigative tool, used both in animal models of acute respiratory distress syndrome (ARDS) [17–19], and in ventilated humans with acute lung injury and ARDS [20–22]. Determination of lung volume using CT has only recently been applied during HFOV [18, 23], with the observation that stepwise pressure increments were associated with volume recruitment and attenuation of CT densities [18]. Importantly, the relatively small oscillatory tidal volumes during HFOV were seen to result in very little peak-to-trough volume change on ultrafast CT scanning [18], confirming that volumetric CT methodology is valid for this mode of ventilation.
The aim of this study was to compare the effect of different methods of lung recruitment on lung volume, gas exchange and distribution of aeration after initiation of HFOV in piglets with lung injury. We compared an escalating pressure strategy conducted over 6 min with a single sustained DI, repeated DIs, and with a pressure strategy in which HFOV was initiated and maintained at a basal P AW 8 cmH2O above that on CMV. We hypothesized that any pressure increments above basal P AW would produce better lung volume, and that a pressure increase of slower onset and longer duration would be associated with greater re-expansion of atelectatic lung units.
Materials and methods
A detailed description can be found in the Electronic Supplementary Material (ESM).
All techniques and procedures were approved by the Animal Experimentation Ethics Committee at the Royal Children’s Hospital, Melbourne. Neonatal piglets were anesthetized, ventilated, catheterized and monitored as described in the ESM. Lung injury was produced by repeated saline lavage, with the endpoint being a PaO2 < 80 mmHg whilst ventilated with an FiO2 of 1.0. CMV was continued at standardized settings [positive end-expiratory pressure (PEEP) 6 cmH2O, ventilator rate 30 inflations/min, Ti at 0.6 s and FiO2 1.0] for 1–2 h to potentiate the lung injury. P AW, tidal volume and compliance of the respiratory system (C RS) were monitored continuously whilst on CMV (Florian Respiratory Monitor, Acutronic Medical Systems, Zug, Switzerland), and peak inspiratory pressure was adjusted to maintain tidal volume at 8–10 mL/kg. The P AW at these settings was noted (P CMV), and from it the basal P AW (P basal) used on HFOV was determined, where P basal = P CMV + 8 cmH2O. This pressure increment above P CMV was chosen on the basis of previous studies in our laboratory in which lesser increases in P AW after initiation of HFOV in the lavaged piglet were associated with poor oxygenation and cardiorespiratory instability [24].
The animal was transferred to the CT scanner, placed supine and thereafter remained stationary throughout the experiment. During an expiratory hold at a PEEP of 6 cmH2O, a scout CT image of the chest was taken (Prospeed CT scanner, GE Healthcare, Rydalmere, Australia), and a single tomogram 1 cm above the level of the diaphragm was chosen, and used for all subsequent imaging. The CT images were acquired at 120 kV, 130 mA, 1 s exposure, and with a slice thickness of 3 mm. CT values were calibrated against lung phantoms of air and water.
Four different HFOV lung recruitment methods were tested on each animal in random order (computer-generated random allocation). The experimental protocol is shown in Fig. 1. Prior to each method, the animal was disconnected to ambient pressure for 15 s, followed by CMV at the above settings for 10 min. A baseline CT image was taken with the lung held at PEEP. HFOV was then commenced at P basal, with a frequency of 6 Hz and an oscillatory amplitude sufficient to produce chest wiggle. One of the four recruitment methods was then applied, followed by a 15 min consolidation period at P basal. Three of the methods involved active recruitment after connection to HFOV, these being: Escalating—step-wise pressure increments of 2 cmH2O per minute for 6 min to a P AW 12 cmH2O above P basal (P peak); Sustained DI—a single inflation sustained for 20 s at P peak with HFOV continuing; and Repeated DI—a short series of six 1 s inflations to P peak. The value of P peak (12 cmH2O above P basal) reflects previous observations in the lavaged lung on HFOV demonstrating adequate recruitment in response to pressures of 10–15 cmH2O above the background P AW [3, 25]. In each case P AW was returned to P basal immediately after the recruitment manoeuvre was completed. The fourth method, Standard recruitment, involved setting P AW immediately at P basal. Further CT images were taken on HFOV prior to active recruitment, and after 1, 5 and 15 min of the consolidation period. Arterial blood gas analyses were performed at baseline after 10 min on CMV, and at the end of the consolidation period. HFOV frequency and amplitude remained unchanged throughout each recruitment method.
Experimental protocol. Depiction of experimental protocol including alterations in P AW made for the different recruitment methods, and timing of CT images and ABG sampling. Each panel shows at left the final 40 s of a 10 min period on standardized CMV settings (in this case P CMV = 15 cmH2O); in the centre connection to HFOV followed by active recruitment of variable duration (shaded light grey); and at right the 15 min consolidation period on HFOV at basal P AW (P basal = 23 cmH2O). Note differing time scales for each sector. The pressure oscillations during HFOV have been attenuated for ease of interpretation. Black arrows indicate timing of CT images (note that one less CT image is taken with Standard recruitment). ABG samples indicated by “×”. a Escalating recruitment with step-wise increments in P AW over 6 min to 35 cmH2O (P peak). b Sustained DI—a single inflation sustained for 20 s at P peak with HFOV continuing. c Repeated DI—a short series of six 1 s inflations to P peak. d Standard—P AW set immediately at P basal with no active recruitment manoeuvre. For each animal the same P basal, and, where relevant, P peak were used for all recruitment methods
Once all recruitment methods had been evaluated, a pressure–volume relationship of the lung was mapped with HFOV continuing. The lung was inflated in a stepwise manner (2 cmH2O per minute), held for 5 min at 24 cmH2O above P CMV to identify total lung capacity (TLC), and deflated in 2 cmH2O decrements to 8 cmH2O below P CMV. CT images were taken after each change in P AW. The animal was then euthanized with intravenous pentobarbitone.
Analysis of the CT images as outlined in the ESM produced a measure of thoracic gas volume (TGV) for each image, expressed as a percentage of the value at TLC. For each recruitment method, a subtraction CT histogram (15 min post-recruitment histogram minus pre-recruitment histogram) was generated (ESM Figure E1) [22] and the change in total volume and gas volume within different pre-defined aeration compartments was calculated (see ESM for definitions).
Data analysis
All comparisons between the four recruitment methods were made using one-way ANOVA, with differences between individual methods identified post hoc using Duncan’s multiple range test. Longitudinal data were compared within each method using a paired t test. A P value <0.05 was considered statistically significant.
Results
Ten piglets (weight 5.1 ± 0.99 kg, mean ± SD) were studied. The animals underwent 12 ± 3.4 saline lavages, after which the PaO2 was 45 ± 15 mmHg, and the PaCO2 was 64 ± 19 mmHg on standardized CMV settings with FiO2 1.0. P CMV in the group was 15 ± 1.5 cmH2O, and thus P basal on HFOV was 23 ± 1.4 cmH2O, and P peak 35 ± 1.5 cmH2O. Baseline lung indices recorded on CMV prior to each recruitment episode were similar between methods (Table 1), with no significant differences in gas exchange, compliance or TGV. Oscillatory amplitudes were also not different between methods (51 ± 8.7 cmH2O (Escalating), 53 ± 8.4 cmH2O (Sustained DI), 51 ± 10 cmH2O (Repeated DI), 53 ± 9.3 cmH2O (Standard), P = 0.89, ANOVA). There was no evidence of destabilisation of the model of lung injury over time, with pre-recruitment oxygenation similar between the first and last recruitment methods for each animal (72 ± 75 vs. 56 ± 19 mmHg, P = 0.50, paired t test), as were the C RS values on CMV (0.47 ± 0.16 vs. 0.46 ± 0.10 mL/cmH2O/kg, P = 0.82). No pneumothoraces occurred during the experimental protocol. A modest fall in mean blood pressure was noted during active recruitment at P peak, although only for the Repeated DI method was the difference from the pre-recruitment value statistically significant (ESM Table E1).
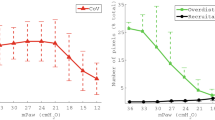
The change in lung volume with the different methods of recruitment is shown in Fig. 2. Escalating recruitment resulted in a TGV at 15 min that was significantly higher than the other methods (77 ± 3.3% of TLC vs. 70 ± 4.2% (Sustained DI), 65 ± 3.5% (Repeated DI), 63 ± 5.1% (Standard); mean ± SEM; P = 0.042, ANOVA; P < 0.05, Duncan’s test). All pressure strategies involving an active recruitment manoeuvre showed an immediate increase in TGV, demonstrated 1 min after recruitment, with little further volume change thereafter (Fig. 1). By contrast, Standard recruitment resulted in a time-dependent increase in volume, with TGV at 15 min being significantly greater than at 1 or 5 min (P = 0.0087 and 0.015, respectively, paired t test).
Thoracic gas volume changes after recruitment. Plot of thoracic gas volume (expressed as a percentage of TLC) on CMV at PEEP 6 cmH2O, after initiation of HFOV at P basal, and 1, 5 and 15 min after recruitment. Mean and SEM. The recruitment period (indicated by the grey bar) varied from 0 s (Standard method) to 6 mins (Escalating method). *TGV higher at 15 min with Escalating recruitment than all other methods; TGV with Standard method lower than Sustained DI; P = 0.0042, ANOVA; P < 0.05, Duncan’s test
Plotting the 15 min TGV values for each recruitment method within the entire PV relationship allowed delineation of the region within which HFOV was ultimately being applied (Fig. 3). Escalating recruitment achieved lung volumes midway between the inflation and deflation limbs of the PV relationship. Repeated DI and Standard recruitment methods, on the other hand, resulted in HFOV being applied on or near the inflation limb.
Final lung volume plotted within the pressure–volume relationship. Plot of pressure–volume relationship of the lung obtained using stepwise pressure increments and decrements on HFOV, with sequential CT images used for determination of TGV (see text for further explanation). The value of TGV after 5 min at P CMV + 24 cmH2O is defined as total lung capacity. The location of TGV values at 15 min post-recruitment for each recruitment method is plotted. Mean and SEM. Differences in TGV between recruitment methods indicated in Fig. 2
The gas volume changes noted with the different recruitment methods were mirrored by changes in oxygenation (Fig. 4). The PaO2 after the 15 min consolidation period was significantly higher with Escalating recruitment than with Repeated DI or Standard methods (307 ± 41 mmHg (Escalating) vs. 159 ± 36 mmHg (Repeated DI) vs. 134 ± 39 mmHg (Standard); mean ± SEM; P = 0.016, ANOVA). No significant differences in PaCO2 measurements were noted between the recruitment methods.
Change in gas exchange with recruitment. Plot of PaO2 (a) and PaCO2 (b) at baseline on standard CMV settings, and at 15 min post-recruitment on HFOV at P basal. Mean and SEM. *PaO2 higher with Escalating recruitment than Standard or Repeated DI recruitment, P = 0.016, ANOVA. No differences were noted in PaCO2 between different recruitment methods
Changes in volume within the previously defined aeration compartments within the CT slice are shown in Fig. 5, both for total volume (panel a) and gas volume (panel b). All recruitment methods resulted in volume loss from the non-aerated compartment, which was less prominent with Standard recruitment (panel a). Non-aerated lung redistributed to the poorly and normally aerated compartments, with the greatest net gain in total volume of normally aerated lung occurring after Escalating recruitment (2.2 ± 0.54 vs. 1.1 ± 0.28 mL (Sustained DI); 0.76 ± 0.23 mL (Repeated DI); 0.82 ± 0.33 mL (Standard); mean ± SEM; P = 0.026, ANOVA). Whilst of lesser magnitude, the pattern of redistribution to poorly and normally aerated lung was similar with the Standard method compared to those involving an active recruitment manoeuvre. These changes are reflected in the topography of the subtraction histograms noted with each method (ESM Figure E2).
Volume change in aeration compartments during recruitment. Plot of change in total volume (a) and gas volume (b) during recruitment for each aeration compartment, comparing 15 min post-recruitment image with pre-recruitment image, both on HFOV at P basal. Mean and SEM. Open bars overdistended (−1,000 to −900 HU); speckled bars normally aerated (−900 to −500 HU); hatched bars poorly aerated (−500 to −100 HU); solid bars non-aerated (−100 to +100 HU). *Greater increase in total volume and gas volume of normally aerated lung with Escalating recruitment than other methods (P = 0.026 and 0.032, respectively, ANOVA). †Less dissipation of non-aerated lung with Standard recruitment compared to other methods (P = 0.027). Gas volume change of overdistended lung trended higher with Escalating recruitment (P = 0.16, ANOVA)
Discussion
This study compared different methods of lung recruitment during the initiation of HFOV in the lung-injured piglet. We found that techniques employing an active pressure strategy (dynamic inflations or escalating recruitment) resulted in greater thoracic gas volume and dissipation of non-aerated lung than initiation of HFOV without active recruitment at a P AW 8 cmH2O above P CMV. Escalating recruitment with a gradual increase in P AW over 6 min was associated with the highest TGV, greatest redistribution to normally aerated lung and best oxygenation, and was the only strategy in which final lung volume was close to that of the deflation limb of the P–V curve. None of the recruitment methods led to unacceptable levels of lung overdistension or hypotension.
The potential advantages of applying HFOV with a high lung volume are well documented. In animal models of lung injury, pressure strategies that demonstrably increase lung volume have been shown to improve oxygenation [3–5, 15, 25, 26], lung function [3, 25, 26] and reduce ventilator-induced lung injury [26]. In human infants, evidence for the benefit of a high lung volume during HFOV is largely circumstantial, with a paucity of direct measurements of volume during HFOV [6, 27], and no systematic lung volume assessments in any randomized controlled trial of HFOV against CMV [8]. Analysis of ventilation strategies used in trials of HFOV in preterm infants does suggest a benefit over CMV if a high lung volume is targeted [2], albeit using indirect indicators of lung volume, in particular oxygenation, as a guide.
Our study compared different techniques of active recruitment on initiation of HFOV, namely sustained dynamic inflations and escalating recruitment. Sustained inflations, consisting of transient application of a high pressure at the airway opening, have been advocated for increasing lung volume during HFOV since its inception [15], but are infrequently used in preterm infants because of concern about barotrauma, impairment of cardiac output and cerebral venous hypertension [1]. Both the pressure increment [3, 25] and the duration are known to contribute to the success of such manoeuvres. We selected two durations of sustained inflation for this study, one known to allow lung volume change to reach completion in the lavaged lung (20 s) [17], and another advocated in the clinical setting in the preterm infant (1 s) [28], in this case repeated six times. These were delivered as dynamic inflations (i.e. with HFOV continuing), which are more effective than static inflations in augmenting lung volume [3].
These techniques of dynamic inflation were compared with escalating recruitment, involving stepwise increases in P AW ultimately to the same P peak. In experimental studies, such pressure increments have been noted to increase lung volume [4, 5, 21] and reverse atelectasis [21]. Escalating pressure strategies have been applied with apparent safety in preterm infants on HFOV [6, 29–31], using a P peak between 5 and 12 cmH2O above the background HFOV P AW. The timing of escalating recruitment in our study (2 cmH2O increments each minute) was similar to that reported in clinical practice [14, 30].
Escalating recruitment over a 6 min period produced the greatest increase in thoracic gas volume 15 min post-manoeuvre, and thus positioned the lung closer to the deflation limb of the pressure–volume relationship. Use of a greater P peak or a longer time interval between pressure increments during recruitment may have moved the point of ventilation even closer to the deflation limb. In clinical practice this is achieved by escalation of P AW until oxygenation no longer improves, or begins to deteriorate, a point deemed to represent TLC [6, 32]. Reduction in P AW thereafter allows HFOV to be applied on the deflation limb near the point of maximal curvature [6]. This technique has been found in both experimental animals and human infants to optimize gas exchange [3–6, 33, 34] and compliance [3, 6], despite a lung volume lower than TLC [3, 33, 35]. Such an approach should also represent the quintessential lung-protective ventilation strategy, avoiding both atelectrauma related to repeated collapse and expansion of unstable alveoli [36], and overdistension related to tidal ventilation occurring near TLC [22]. These clear theoretical benefits have translated to convincing reductions in lung injury with HFOV after lung recruitment in some laboratory studies [34, 37], but not others [35, 38].
The findings of the present study highlight the fundamental concept that both pressure and time are important determinants of the effectiveness of pressure recruitment strategies during HFOV, and it is inappropriate to consider one without the other. Walsh and coworkers [25] were the first to investigate the pressure–time interplay during recruitment on HFOV in the lavaged rabbit lung, finding that static sustained inflations of 3 s duration produced minimal volume change or oxygenation effect at any pressure increment up to 15 cmH2O above baseline P AW (P peak ~ 27 cmH2O). We found on the other hand that brief sustained inflations of 1 s duration (P peak of ~35 cmH2O) were associated with reversal of atelectasis beyond that achieved using a standard pressure strategy. The disparity in these findings may be explained by the different peak pressures used, and additionally the maintenance of the oscillatory pressure waveform, i.e. use of dynamic rather than static inflation, in our study [3].
The considerable additional benefit of stepwise recruitment in our study suggests altered volume behavior and a different distribution of aeration can be achieved with slow recruitment starting at a pressure well below P peak. Odenstedt and coworkers [39] report a similar finding in comparing sustained static inflations (40 cmH2O, 3 periods of 30 s) with high-level pressure control ventilation (P peak 40 cmH2O, 3 periods of 30 s) and a slow lower pressure manoeuvre (volume control ventilation, P peak ~ 27 cmH2O, 15 min) in saline-lavaged pigs, with better oxygenation and compliance in the dependent regions noted after slow recruitment despite the considerably lower peak pressure in this group.
The crossover design used in this study does bring with it the possibility of error related to change in the lavage model over time, and incomplete return to baseline after each method of recruitment. For this reason the recruitment methods were applied in random order, and lung volume history was standardised through disconnection to ambient pressure followed by 10 min of conventional ventilation at uniform settings. Analysis of baseline lung function on CMV did not show any systematic differences between recruitment methods, nor any obvious change in the model during the entire study period.
Conclusion
Escalating recruitment produced the greatest increase in lung volume and resolution of atelectasis, and is recommended for lung volume recruitment upon initiation of HFOV in infants with parenchymal lung disease.
References
Froese AB, Kinsella JP (2005) High-frequency oscillatory ventilation: lessons from the neonatal/pediatric experience. Crit Care Med 33:S115–S121
Cools F, Henderson-Smart DJ, Offringa M, Askie LM (2009) Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev CD000104
Byford LJ, Finkler JH, Froese AB (1988) Lung volume recruitment during high-frequency oscillation in atelectasis-prone rabbits. J Appl Physiol 64:1607–1614
Brazelton TB III, Watson KF, Murphy M, Al Khadra E, Thompson JE, Arnold JH (2001) Identification of optimal lung volume during high-frequency oscillatory ventilation using respiratory inductive plethysmography. Crit Care Med 29:2349–2359
Gothberg S, Parker TA, Griebel J, Abman SH, Kinsella JP (2001) Lung volume recruitment in lambs during high-frequency oscillatory ventilation using respiratory inductive plethysmography. Pediatr Res 49:38–44
Tingay DG, Mills JF, Morley CJ, Pellicano A, Dargaville PA (2006) The deflation limb of the pressure–volume relationship in infants during high-frequency ventilation. Am J Respir Crit Care Med 173:414–420
Rimensberger PC, Pristine G, Mullen BM, Cox PN, Slutsky AS (1999) Lung recruitment during small tidal volume ventilation allows minimal positive end-expiratory pressure without augmenting lung injury. Crit Care Med 27:1940–1945
van Kaam AH, Rimensberger PC (2007) Lung-protective ventilation strategies in neonatology: what do we know–what do we need to know? Crit Care Med 35:925–931
Courtney SE, Durand DJ, Asselin JM, Hudak ML, Aschner JL, Shoemaker CT (2002) High-frequency oscillatory ventilation versus conventional mechanical ventilation for very-low-birth-weight infants. N Engl J Med 347:643–652
Johnson AH, Peacock JL, Greenough A, Marlow N, Limb ES, Marston L, Calvert SA (2002) High-frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med 347:633–642
Froese AB, Butler PO, Fletcher WA, Byford LJ (1987) High-frequency oscillatory ventilation in premature infants with respiratory failure: a preliminary report. Anesth Analg 66:814–824
Gerstmann DR, Minton SD, Stoddard RA, Meredith KS, Monaco F, Bertrand JM, Battisti O, Langhendries JP, Francois A, Clark RH (1996) The Provo multicenter early high-frequency oscillatory ventilation trial: improved pulmonary and clinical outcome in respiratory distress syndrome. Pediatrics 98:1044–1057
Thome U, Kossel H, Lipowsky G, Porz F, Furste HO, Genzel-Boroviczeny O, Troger J, Oppermann HC, Hogel J, Pohlandt F (1999) Randomized comparison of high-frequency ventilation with high-rate intermittent positive pressure ventilation in preterm infants with respiratory failure. J Pediatr 135:39–46
Rimensberger PC, Beghetti M, Hanquinet S, Berner M (2000) First intention high-frequency oscillation with early lung volume optimization improves pulmonary outcome in very low birth weight infants with respiratory distress syndrome. Pediatrics 105:1202–1208
Kolton M, Cattran CB, Kent G, Volgyesi G, Froese AB, Bryan AC (1982) Oxygenation during high-frequency ventilation compared with conventional mechanical ventilation in two models of lung injury. Anesth Analg 61:323–332
Froese AB, McCulloch PR, Sugiura M, Vaclavik S, Possmayer F, Moller F (1993) Optimizing alveolar expansion prolongs the effectiveness of exogenous surfactant therapy in the adult rabbit. Am Rev Respir Dis 148:569–577
Neumann P, Berglund JE, Fernandez ME, Magnusson A, Hedenstierna G (1998) Dynamics of lung collapse and recruitment during prolonged breathing in porcine lung injury. J Appl Physiol 85:1533–1543
Luecke T, Meinhardt JP, Herrmann P, Weisser G, Pelosi P, Quintel M (2003) Setting mean airway pressure during high-frequency oscillatory ventilation according to the static pressure–volume curve in surfactant-deficient lung injury: a computed tomography study. Anesthesiology 99:1313–1322
Albaiceta GM, Luyando LH, Parra D, Menendez R, Calvo J, Pedreira PR, Taboada F (2005) Inspiratory vs. expiratory pressure–volume curves to set end-expiratory pressure in acute lung injury. Intensive Care Med 31:1370–1378
Pelosi P, Cadringher P, Bottino N, Panigada M, Carrieri F, Riva E, Lissoni A, Gattinoni L (1999) Sigh in acute respiratory distress syndrome. Am J Respir Crit Care Med 159:872–880
Albaiceta GM, Taboada F, Parra D, Luyando LH, Calvo J, Menendez R, Otero J (2004) Tomographic study of the inflection points of the pressure–volume curve in acute lung injury. Am J Respir Crit Care Med 170:1066–1072
Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, Slutsky AS, Gattinoni L, Ranieri VM (2007) Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 175:160–166
Mulreany DG, Simon BA, Murphy KJ, Easley RB (2009) Volumetric xenon-CT imaging of conventional and high-frequency oscillatory ventilation. Acad Radiol 16:718–725
Mills JF, Davis CE, Mazzolini A, Dargaville PA (2000) Relationship between chest wall movement and tidal volume during high frequency oscillatory ventilation. Pediatr Res 47:369A
Walsh MC, Carlo WA (1988) Sustained inflation during HFOV improves pulmonary mechanics and oxygenation. J Appl Physiol 65:368–372
McCulloch PR, Forkert PG, Froese AB (1988) Lung volume maintenance prevents lung injury during high frequency oscillatory ventilation in surfactant-deficient rabbits. Am Rev Respir Dis 137:1185–1192
Thome U, Topfer A, Schaller P, Pohlandt F (1998) Effects of mean airway pressure on lung volume during high-frequency oscillatory ventilation of preterm infants. Am J Respir Crit Care Med 157:1213–1218
Moriette G, Paris-Llado J, Walti H, Escande B, Magny JF, Cambonie G, Thiriez G, Cantagrel S, Lacaze-Masmonteil T, Storme L, Blanc T, Liet JM, Andre C, Salanave B, Breart G (2001) Prospective randomized multicenter comparison of high-frequency oscillatory ventilation and conventional ventilation in preterm infants of less than 30 weeks with respiratory distress syndrome. Pediatrics 107:363–372
Kalenga M, Battisti O, Francois A, Langhendries JP, Gerstmann DR, Bertrand JM (1998) High-frequency oscillatory ventilation in neonatal RDS: initial volume optimization and respiratory mechanics. J Appl Physiol 84:1174–1177
De Jaegere A, van Veenendaal MB, Michiels A, van Kaam AH (2006) Lung recruitment using oxygenation during open lung high-frequency ventilation in preterm infants. Am J Respir Crit Care Med 174:639–645
de Waal K, Evans N, van der LJ, van Kaam A (2009) Effect of lung recruitment on pulmonary, systemic, and ductal blood flow in preterm infants. J Pediatr 154:651–655
Lachmann B (1992) Open up the lung and keep the lung open. Intensive Care Med 18:319–321
Goddon S, Fujino Y, Hromi JM, Kacmarek RM (2001) Optimal mean airway pressure during high-frequency oscillation: predicted by the pressure–volume curve. Anesthesiology 94:862–869
Muellenbach RM, Kredel M, Said HM, Klosterhalfen B, Zollhoefer B, Wunder C, Redel A, Schmidt M, Roewer N, Brederlau J (2007) High-frequency oscillatory ventilation reduces lung inflammation: a large-animal 24-h model of respiratory distress. Intensive Care Med 33:1423–1433
Rimensberger PC, Pache JC, McKerlie C, Frndova H, Cox PN (2000) Lung recruitment and lung volume maintenance: a strategy for improving oxygenation and preventing lung injury during both conventional mechanical ventilation and high-frequency oscillation. Intensive Care Med 26:745–755
Halter JM, Steinberg JM, Schiller HJ, Dasilva M, Gatto LA, Landas S, Nieman GF (2003) Positive end-expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/derecruitment. Am J Respir Crit Care Med 167:1620–1626
Imai Y, Nakagawa S, Ito Y, Kawano T, Slutsky AS, Miyasaka K (2001) Comparison of lung protection strategies using conventional and high-frequency oscillatory ventilation. J Appl Physiol 91:1836–1844
van Kaam AH, De Jaegere A, Haitsma JJ, van Aalderen WM, Kok JH, Lachmann B (2003) Positive pressure ventilation with the open lung concept optimizes gas exchange and reduces ventilator-induced lung injury in newborn piglets. Pediatr Res 53:245–253
Odenstedt H, Lindgren S, Olegard C, Erlandsson K, Lethvall S, Aneman A, Stenqvist O, Lundin S (2005) Slow moderate pressure recruitment maneuver minimizes negative circulatory and lung mechanic side effects: evaluation of recruitment maneuvers using electric impedance tomography. Intensive Care Med 31:1706–1714
Acknowledgments
This study was supported by a Project Grant, a Trainee Research Scholarship (AP), and a Career Development Grant (PAD) from the Murdoch Childrens Research Institute, and an Australian National Health and Medical Research Council Medical Postgraduate Research Scholarship (DGT). We thank Magdy Sourial, Omar Kamlin, Melissa Dodsworth, Paul Ekert and the Royal Children’s Hospital CT radiographers for their assistance in the completion of this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pellicano, A., Tingay, D.G., Mills, J.F. et al. Comparison of four methods of lung volume recruitment during high frequency oscillatory ventilation. Intensive Care Med 35, 1990–1998 (2009). https://doi.org/10.1007/s00134-009-1628-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1628-8